Oxidative phosphorylation in Mitochondria
- Understand how electrons are transferred in the respiratory chain and how the energy is harvested
- Understand how electrons from different reactions are funnelled into the respiratory chain
- To understand the chemiosmotic mechanism of ATP synthesis
- Appreciate the nanomachine ATP synthase
- Electron transfer along redox centers
- Ubiquinone as a mobile electron carrier
- Redox potential
- Chemiosmotic ATP synthesis
- Shuttle systems
- Uncoupling
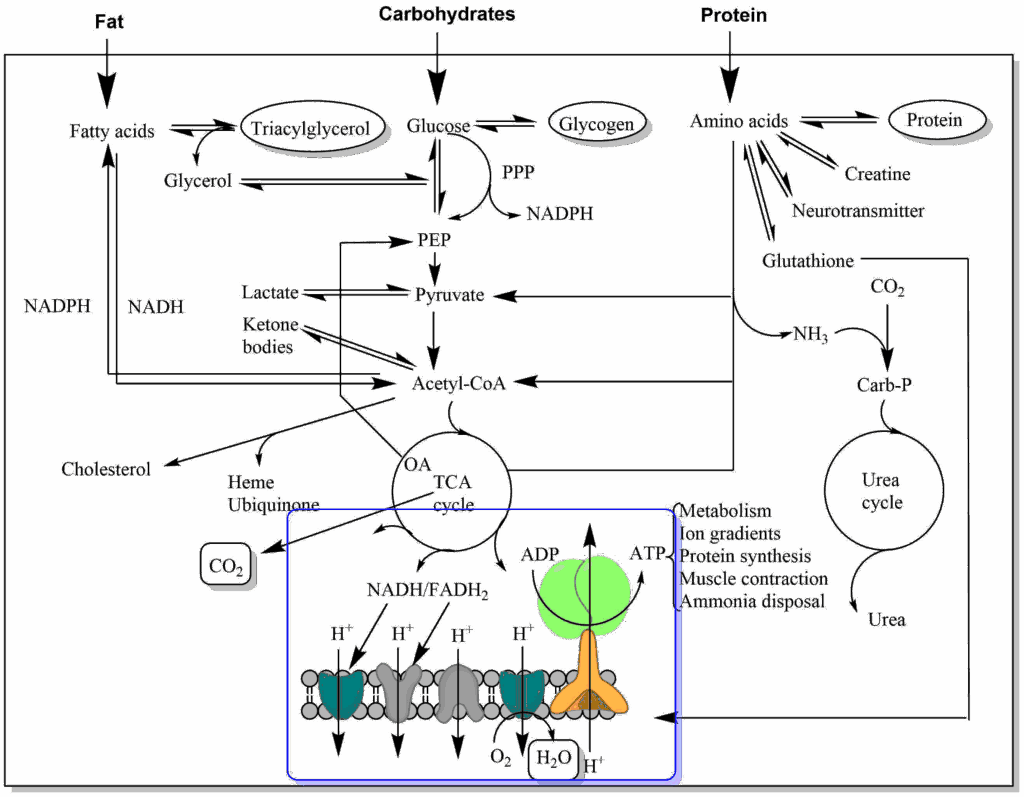
Thus far we have followed the metabolism of glucose through glycolysis and the TCA cycle and witnessed the breakdown of the carbon skeleton to six molecules of carbon dioxide. In these metabolic pathways we oxidised metabolic intermediates and stored electrons in coenzymes so that we could eventually release carbon dioxide. Now we want to explore what happens to the electrons and how water is generated and the energy used to make ATP.
Remember, oxidation is a reaction where a loss of electrons occurs, while reduction is accompanied by a gain of electrons. The electrons from the TCA cycle were stored in the molecule NADH or FADH2. In Redox reactions electrons flow from the partner that has a stronger propensity to donate electrons to the partner that has a propensity to accept electrons. We have seen that intermediates of glycolysis and the TCA cycle can donate electrons and that these are transferred to NAD+ and FAD. These compounds still carry most of the energy associated with the oxidation reaction and the questions arises: How do we harvest the energy of electrons (also called reducing equivalents)? The place where we harvest this energy is called the respiratory chain or electron transport chain (ETC). It is called the respiratory chain, because the final acceptor of the electrons is oxygen. Before we look into the proteins of the respiratory chain let us have a look at some molecules that are suitable to transfer electrons (Table 1). We also need to have a scale about the propensity to donate or accept electrons. This is called the Redox Potential (Table 1 and see chapter 3). In Table 1 you may note that the NADH/NAD+ pair has a more negative redox potential than the malate/oxaloacetate pair. This explains why the last TCA cycle reaction actually favours formation of malate and only small amounts of oxaloacetate are available for citrate formation (see chapter 9).
|
Redox pair
|
Number of electrons transferred
|
Redox Potential (V)
|
|---|---|---|
|
NADH/NAD+, NADPH/NADP+
|
2
|
-0.32
|
|
FMN (complex I)
|
2
|
-0.30
|
|
Glyceraldehyde-3-phosphate /1,3-bisphosphoglycerate
|
2
|
-0.29
|
|
FADH2/FAD
|
2
|
-0.219
|
|
Lactate/Pyruvate
|
2
|
-0.185
|
|
Malate/Oxaloacetate
|
2
|
-0.166
|
|
Succinate/Fumarate
|
2
|
+0.03
|
|
Ubiquinol/Ubiquinone
|
2
|
+0.06
|
|
Cytochrome c
|
1
|
+0.254
|
|
Cytochrome a
|
1
|
+0.290
|
|
1/2 O2/H2O
|
2
|
+0.816
|
Let us have a look at molecules (or co-factors) that transfer electrons in the respiratory chain (Fig. 2).
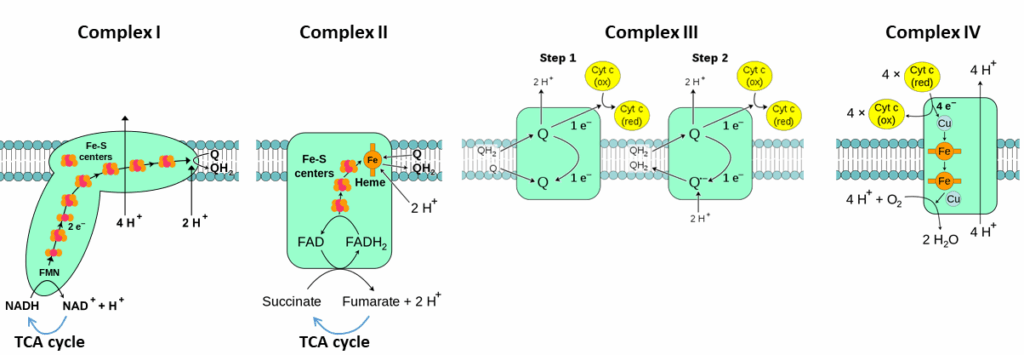
There are only five principal types of electron transfer complexes in the respiratory chain. Namely, Flavin mononucleotide (FMN), iron-sulphur centres, ubiquinone (or coenzyme Q), cytochromes containing heme-iron and copper-ions that are complexed by cysteine and histidine residues (Fig. 2). FMN is a shorter version of FAD, the redox reaction of which we discussed in chapter 9. Thus FMN becomes FMNH2 after accepting two electrons and two protons. In all iron complexes electron transfer occurs because of a reversible change from Fe2+ to Fe3+. The redox reaction of coenzyme Q is a two-step reaction converting ubiquinol to ubiquinone via a semi-quinone radical with an unpaired electron.
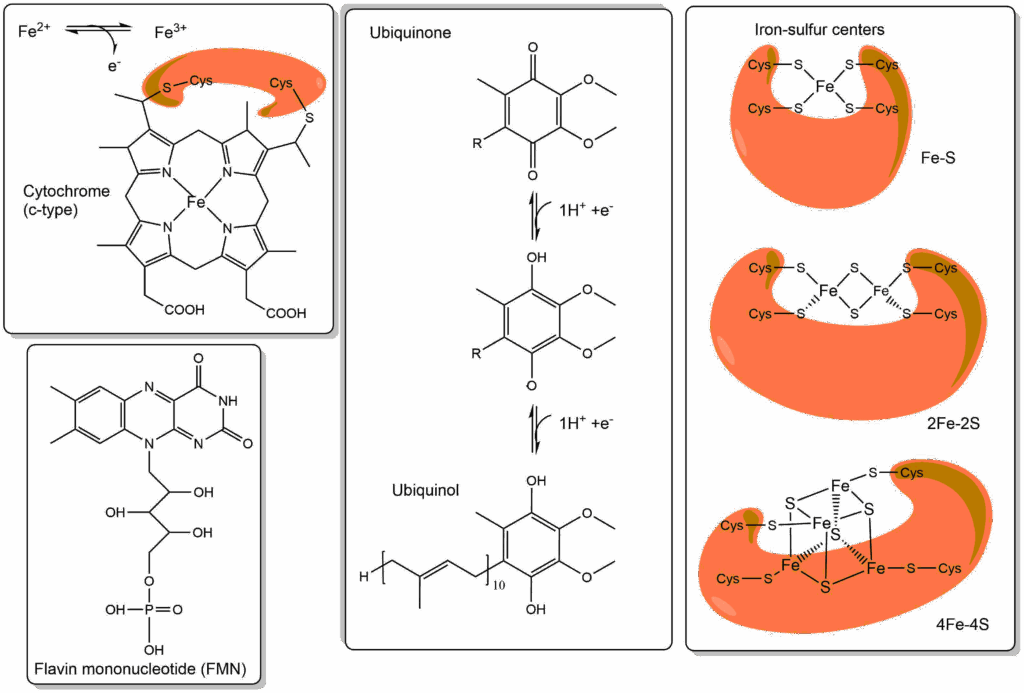
From table 1 you can see that organic metabolites like to donate electrons, while cytochromes are more likely to accept electrons, ubiquinone is somewhere in the middle. The iron-sulphur centres can actually not be studied in isolation but redox potentials can be measured by spectroscopic methods, even though several are found in a single protein complex of the respiratory chain. In general, the redox potentials of iron-sulphur clusters sit between NADH and ubiquinone. Cytochromes, Iron-sulphur centres and copper centres are protein bound molecules (or cofactors), while ubiquinone is a mobile, membrane embedded electron carrier. Please note the long ten-repeat isoprenoid lipid anchor of ubiquinone, which allows it to “swim” in the lipid bilayer. What you can now recognise is a series of electron transferring molecules that have slightly different redox potentials. If you line these up in the order of decreasing redox potential you have a “chain” of “electron transporters”, the electron transport chain (ETC, Fig. 3).
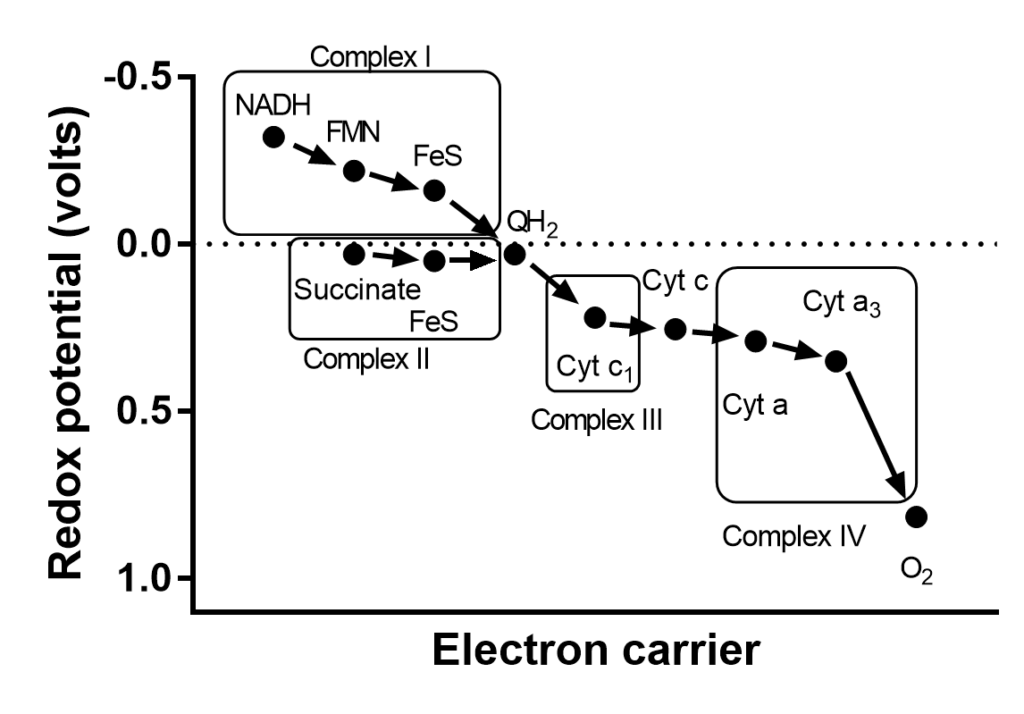
With the exception of the mobile coenzyme Q, electron carriers are firmly embedded in proteins. There are four protein complexes in the mitochondrial membrane, which are labelled complex I,II,III and IV (Fig. 3 and 4).
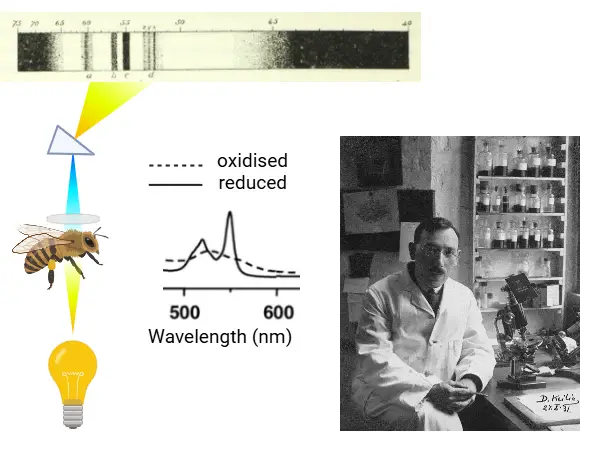
How were cytochromes discovered? Charles McMunn was the first to describe a specific pigment in muscle in the 1880’s. The pigment was colorful when reduced but was pale when oxidised. His work was discredited at the time by German biochemist Felix Hoppe-Seyler, who thought it was an effect generated by hemoglobin. McMunn’s idea was taken up again by David Keilin who looked at light absorption in different organisms and tissues, particularly in insects. In 1925 he discovered distinctive bands in a variety of tissues and organisms and called these cytochromes. He used a special spectrometer, where light could be passed through muscle tissue and analysed for absorption at specific wavelengths.
In yeast cell suspensions he could show that bands were visible when the suspension was not aerated. When air was bubbled through, the bands disappeared leading to the conclusion that oxidised cytochromes had no color, while reduced cytochromes have. Addition of potassium cyanide results in the reduction of all cytochromes, because it stops the transfer of electrons to oxygen (see exercise Oxidative phosphorylation). The color changes form the basis for detailed investigations of the respiratory chain by spectroscopy, because the different complexes of the respiratory chain have spectra that are slightly different.
Why do we have a pool of Ubiquinone? In addition to complex I and II, ubiquinone acts as an acceptor of electrons from variety of sources (Fig. 5).
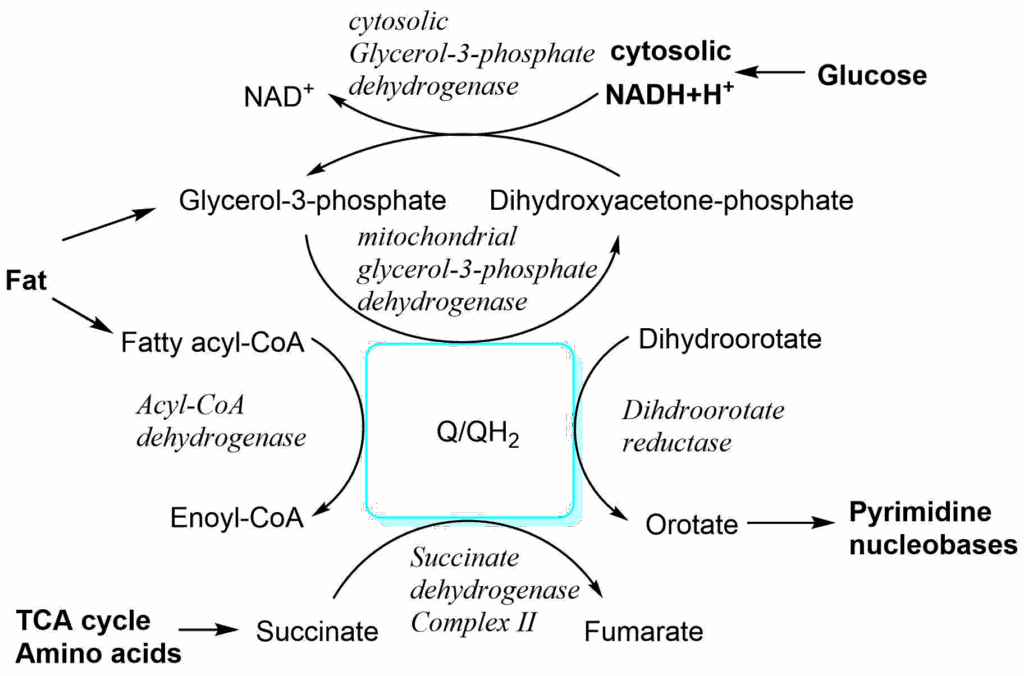
This allows flexible connection of nutrient oxidation to the respiratory chain. While most of these reactions take place in the matrix of mitochondria, NADH generated in the cytosol can be used to reduce dihydroxyacetone-phosphate to glycerol-3-phosphate. The latter can diffuse to the intermembrane space, where the reverse reaction occurs and electrons are transferred to ubiquinone generating ubiquinol.
Complexes of the respiratory chain form larger super-complexes. In particular complex I, III and IV are close to each other, while complex II is separate (Fig. 6). Why could this be important?
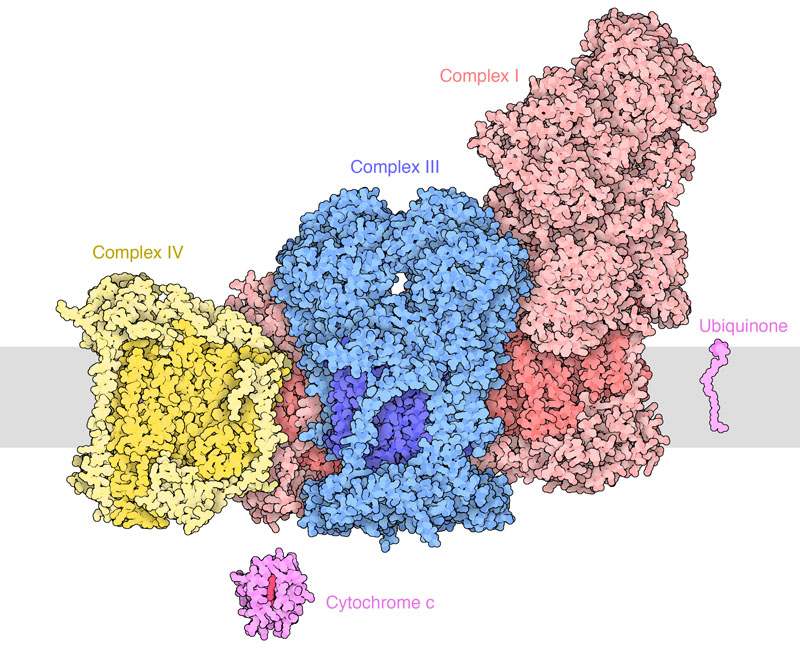
As we will learn in chapter 17, the ubiquinone semi-radical can transfer electrons to oxygen forming oxygen radicals. Channeling of QH2 from complex I to complex III prevents exposure to oxygen. Radicals can damage cells over long periods. Carbohydrates feed most of their electrons into complex I, while fat feeds more electrons into complex II. Over-consumption of fat and sugar together can result in a traffic jam where ubiquinol from complex II may not be able to offload electrons and therefore reacts with oxygen. The resulting damage to cells can cause insulin resistance, beta-cell damage and diabetes. The combination of sugar + fat might be particularly unhealthy for cells. However, the significance of ubiquinone channeling is highly disputed.
Ref: Fedor JG, Hirst J, Mitochondrial supercomplexes do not enhance catalysis by quinone channeling. PMCID: PMC6125145
We now return to the discussion of the respiratory chain itself and ask: Where do we store the energy that is released during step-by-step electron transfer? Closer inspection of Fig. 4 shows a vectorial transport of protons from the mitochondrial matrix to the intermembrane space by complexes I, III and IV. This is literally like charging a battery, because charges are separated by an insulator (membrane). The membrane potential is inside negative, because protons are pumped out of the matrix. In addition it generates a small pH gradient (Fig. 7). The pH gradient (expressed in mV) combined with the membrane potential (expressed in mV) resulting from the vectorial transport of protons is called the proton motive force (PMF). The PMF quantifies the energy that was stored in electrons derived from nutrient metabolism. This energy has now been converted into an electrochemical gradient.
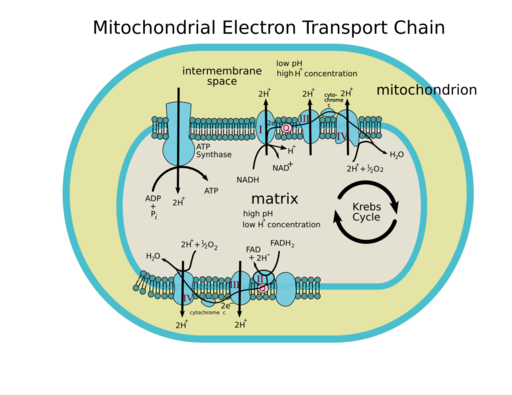
We have now finally arrived at the conversion of nutrient energy into ATP formation. The proton motive force is used in the nanomachine ATP synthase to make ATP. How does this work? The word nanomachine was used deliberately here, because the ATP synthase converts the PMF into a rotation. The head of the ATP synthase has three repeats of alpha and beta subunits (the head is also called F1). ADP and phosphate can bind to an active site between the alpha and beta subunit. The head of the ATP synthase is held in place by a large arm that attaches to the side and top of the hetero-hexamer. In the centre of the hexamer is an asymmetric stalk that is attached to a rotating ring in the membrane. When the ring in the membrane rotates so does the stalk. Due to the asymmetry of the stalk, rotation will change the association between each pair of the alpha and beta subunits. In fact each of the three pairs is in a different state, namely (1) empty, (2) ADP and Pi bound, (3) ATP formed. When the stalk allows the alpha and beta subunits to tighten around ADP and Pi, the two molecules fuse and water is released. Magnesium is required as a cofactor in the reaction. In the confined space of the ATP synthase, the condensation reaction is close to equilibrium. The rotational energy is actually used to expel ATP in the next step. Thus, mechanical energy is converted into chemical energy!
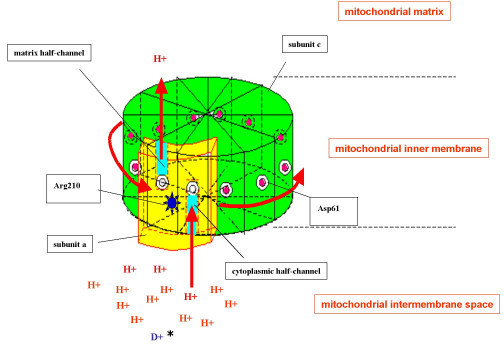
Where does the rotational energy come from? The membrane embedded part (called F0) has 10-14 identical subunits, depending on the species and subtype of ATPase (Fig. 8). Each subunit has a binding site for a proton in the centre of the membrane. There is a tunnel that allows protons to reach the centre of the membrane from the intermembrane space. The tunnel to leave the binding site sits slightly apart and as a result each proton-binding subunit must revolve around to offload its proton after a full rotation. In one rotation as many protons are transferred as the number of F0 subunits. Most textbooks use 12 as a consensus number, i.e. 4 protons per ATP formed because each of the three alpha/beta pairs of the F1 part goes through the three steps of ATP synthesis per rotation.
The generation of ATP by ATP synthase is called the chemiosmotic mechanism of ATP synthesis. The term points to the pH gradient (a chemical concentration gradient that results in osmotic pressure) as the main driving force for the generation of ATP. It is important to note that the pH gradient is actually quite small, most of the energy is in the separation of charges, more like a battery. Chemiosomotic is perhaps not the best term but is used across the literature. Peter Mitchell received the Nobel prize for the chemiosmotic mechanism of ATP synthesis. It is proven beyond doubt today, but was very controversial at the time, because the well-established substrate-level phosphorylation mechanism developed from research on glycolysis was in the forefront of many minds as the principal mechanism of ATP synthesis.

How many ATP can be generated from a molecule of glucose?
Fig. 4 shows that complex I pumps four protons across the membrane when two electrons are passed to ubiquinone. That is equivalent to the generation of 1 ATP. If you add up all protons about 3 ATP can be synthesised when NADH+H+ provides electrons to the respiratory chain, but only 2 ATP are synthesised from FADH2. These numbers are the upper limits, because other process can use up the proton gradient such as ATP translocation and phosphate translocation. As a result, 2.5 and 1.5 ATP are used generally. Calculate the number of NADH and FADH2 generated by oxidising a molecule of glucose and then calculate the maximum number of ATP (include ATP generated during glycolysis) generated by a molecule of glucose.
We are still missing some important details. First, all the ATP has been created in the matrix of the mitochondria. Most of the ATP is, however, required inside the cytosol (e.g. muscle work, ion transport, protein biosynthesis). If the ATP was stuck inside mitochondria, metabolism would stop very quickly because of lack of ADP.
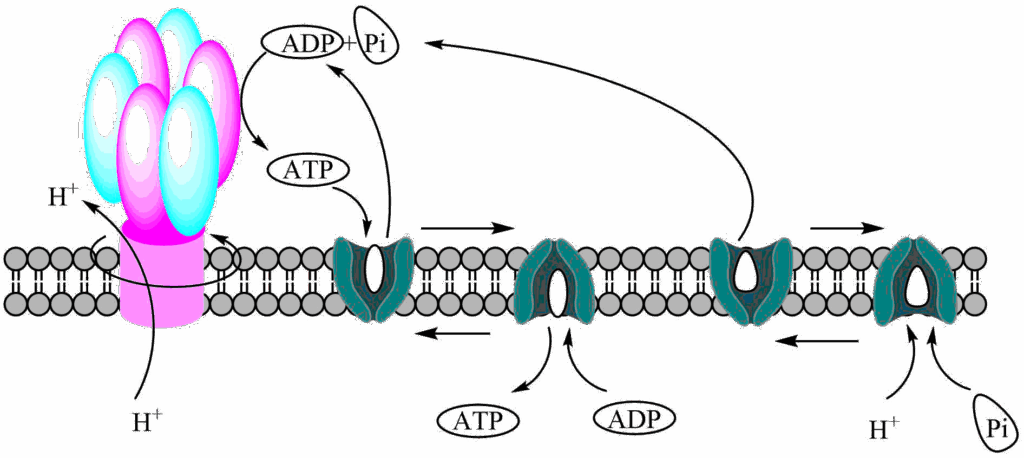
A transporter is required to exchange ATP and ADP (Fig. 9). Please note that ATP has 3 negative charges, while ADP has 2. As a result export of ATP and import of ADP is favoured because of the prevailing membrane potential, at the same time it dissipates some of the PMF. The transporter is known as the ADP/ATP-translocase or carrier. We also need a phosphate carrier to bring phosphate into the matrix, which occurs in symport with protons (or antiport of OH–) and therefore dissipates some of the proton gradient.
Tempeh is a traditional fermented soy bean product from Indonesia. Tempeh bongkrek is a variant of this product made with additional coconut dregs. This has led to fatal food poisoning when the the coconut dregs were contaminated with the bacterium Burkholderia gladiolis. The bacterium produces bongrekic acid, which blocks the ADP/ATP carrier resulting in death due to lack of ATP production.
The second detail we are missing is the fate of NADH generated during glycolysis. The NADH production during glycolysis is subject to metabolic coupling and will slow down the pathway if NAD+ is not reformed. We learned that in the absence of oxygen (fermentation or muscle metabolism when sprinting), NAD+ was regenerated by formation of lactate (chapter 7). In muscle, this can only proceed for a limited time before lactate accumulation becomes too high. For long periods of exercise NADH must be converted back to NAD+ using the respiratory chain, but how does NADH get into mitochondria? Revisit Fig. 5 and discover how the cytosolic and mitochondrial isoforms of glycerol-3-phosphate dehydrogenase work together to transfer the two electrons (reducing equivalents) from NADH to the respiratory chain. This is known as the glycerol-phosphate shuttle. Better known, although not present in all tissues, is the more complicated malate-aspartate shuttle (Fig. 10).
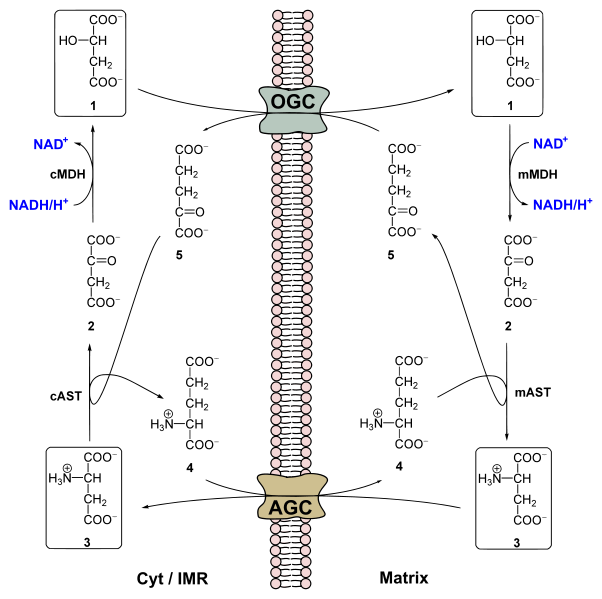
The shuttle involves two transporters, the oxoglutarate carrier (OGC) and the aspartate-glutamate carrier (AGC) and four enzymes (cytosolic and mitochondrial malate dehydrogenase; cytosolic and mitochondrial aspartate transaminase). Closer inspection of Fig. 10 shows that all elements are recycled, apart from the net transfer of NADH into the mitochondrial matrix in exchange of NAD+. The shuttle can be viewed as a large enzymatic complex that catalyses the exchange of NADH vs NAD+ across the mitochondrial membrane. You may notice the swap of a keto-group and an amino-group in the shuttle associated enzymatic reaction. We will learn more about this transaminase reaction in chapter 14.
What would happen if you could short-circuit the mitochondrial battery?
There are two ways to short-circuit the mitochondrial electron transport chain. One is by specific chemical compounds, for instance 2,4-dinitrophenol (Fig. 11); the second is through expression of the uncoupling protein (Fig. 11). Both translocate protons across the inner mitochondrial membrane. This uncouples the electron transport chain from ATP production, because protons can flow back without going through ATP synthase. You can compare it with inflating a bicycle tube that has a puncture.
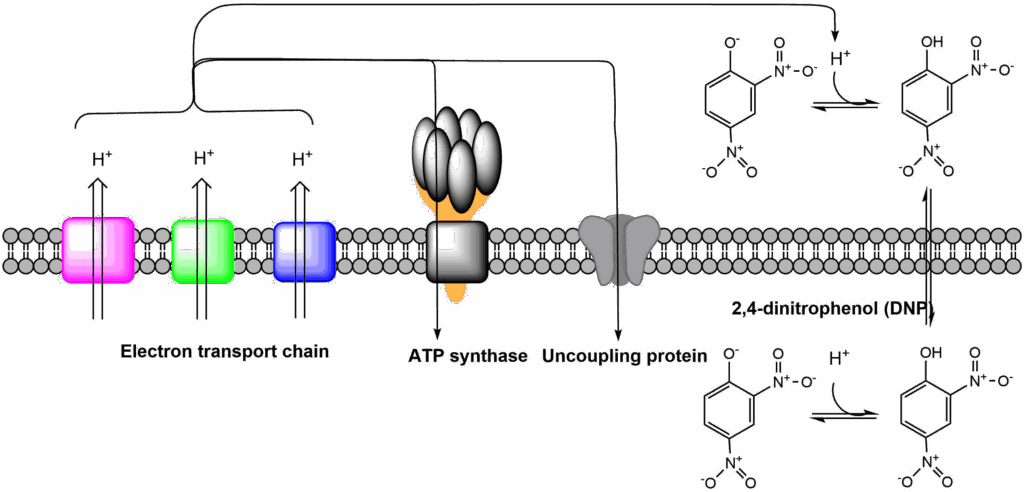
This process is important when thinking about nutrition and obesity. When protons are returned to the matrix without generation of ATP, heat is generated. This process is very important in newborns and babies, which do not exercise to generate body heat. Babies express uncoupling protein in brown adipose tissue (it is called brown because it has more mitochondria than normal adipose tissue and therefore has a brown colour). In newborns brown adipose tissue is found around inner organs, particularly the kidneys and the back of the body, areas which are easily exposed to cold temperature. In adults, brown adipose tissue almost disappears and is replaced by shivering thermogenesis, but subcutaneous adipose tissue can induce expression of uncoupling protein after exposure to cold temperatures (Fig. 12). This process is called formation of beige adipose tissue or browning. A large amount of research is conducted to understand the mechanisms of beige adipose tissue induction. Triggering these mechanisms would result in burning of nutrients to generate heat instead of storing them as fat. 2,4-Dinitrophenol (DNP), was widely prescribed in the 1930s as a weight loss drug. The treatment is very effective but overdosing resulted in heart failure due to reduced ATP production. Brain and heart use more ATP than any other organ, overdosing of DNP cause damage to these organs. Thus DNP is not safe as an anti-obesity drug, because its therapeutic window is too small. Research is conducted to identify safe mitochondrial uncoupling reagents, which cannot be overdosed and natural compounds that induce expression of uncoupling proteins.
Compare and contrast!
You have encountered ATP production during glycolysis and through ATP synthase. Compare the two mechanisms. What is the fundamental difference? Which process requires oxygen and which one does not?
In glycolysis ADP is phosphorylated by a phospho-organic intermediate of the glycolytic pathway. In the ATP synthase ADP and phosphate are ligated by proximity achieved through mechanical forces. The mechanical forces are derived from the proton motive force. Glycolysis can run in the absence of oxygen through the production of lactate and still produce ATP. Due to the coupling between respiratory chain and ATP synthase, ATP generation stops in the absence of oxygen.
Metabolic coupling occurs between several elements of mitochondrial metabolism and ATP synthesis. Explain how these pathways are connected and why they would all stop if one of them stops.
The TCA cycle generates NADH. Without regeneration of NAD it has no substrate for dehydrogenase reactions. NADH feeds electrons into the respiratory chain. Without NADH no electron flow. The electron flow through the respiratory chain is used to pump protons out of mitochondria, generating a proton motive force (PMF). Without PMF not ATP synthesis. If there is no use for ATP, the PMF will build up slowing down the respiratory chain, which in turn will slow down the TCA cycle.
Explain the concept of Redox potential and how it applies to the respiratory chain.
The Redox potential refers to the propensity of a molecule to accept or donate electrons.
The Redox centers of the electron transport chain (ETC) have a different propensity to donate or accept electrons. The centers of the ETC are ordered by (mathematically) increasing Redox potential. From more negative (likes to donate) to more positive (likes to accept).
Why are transporters essential to integrate cytosolic and mitochondrial metabolism?
ATP is generated inside mitochondria, but mainly used in the cytosol. The ADP/ATP carrier exchanges ADP and ATP between cytosol and mitochondria. The phosphate carrier catalyses the entry of phosphate into mitochondria, which is required for the formation of ATP.
Compare fig. 3 and 4 and make a case why complex II does not translocate protons.
The redox potential of the succinate/fumarate pair is very close to the redox potential of the Q/QH2 pair. There is not enough energy available for proton pumping.
The electron transport chain accepts electrons from NADH, FADH2 and some specific redox reactions.
- Electrons are passed between redox centers of increasing redox potential, eventually reducing oxygen to form water.
- The energy derived from these redox reactions is used to pump protons across the intramitochondrial membrane. This generates a proton motive force (PMF) comprised of a pH-gradient (inside alkaline) and a membrane potential (inside negative).
- The PMF is used to drive the rotation of ATP synthase, which condenses ADP and phosphate.
- Transfer of NADH, ATP, ADP and other metabolites into and out of the mitochondrial matrix requires sophisticated shuttle systems.
- Uncoupling of ATP synthesis from the respiratory chain dissipates the PMF without generation of ATP and generates heat.
Review the elements of the respiratory chain by watching the video below.
- By the author using ChemDraw
- By the author using ChemDraw
- By the author using ChemDraw
- Derived from Fvasconcellos 23:51, 9 September 2007 (UTC) [Public domain], via Wikimedia Commons; Fvasconcellos 22:14, 29 September 2007 (UTC) [Public domain], via Wikimedia Commons; Fvasconcellos 02:19, 19 September 2007 (UTC) [Public domain], via Wikimedia Commons; Fvasconcellos 01:21, 10 October 2007 (UTC) [Public domain], via Wikimedia Commons
- By the author using ChemDraw
- PDB-101: Molecule of the Month: Respiratory Supercomplex
- User:Rozzychan [CC BY-SA 2.5 (https://creativecommons.org/licenses/by-sa/2.5)], via Wikimedia Commons
- https://openi.nlm.nih.gov/detailedresult.php?img=PMC1808445_1742-4682-4-9-1&req=4; License CC BY 2.0.
- By the author using ChemDraw
- Yikrazuul [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], from Wikimedia Commons
- By the author using ChemDraw
- Hg6996 [Public domain], from Wikimedia Commons