11 Digestion and transport of dietary lipids
- Understanding of the role of fat in energy metabolism.
- Have an overview of how fat is digested and then used to generate energy.
- Understand the process of beta-oxidation.
- Understand what ketone bodies are and when they are being produced.
- Understand how ethanol metabolism is linked to fat metabolism.
- Dispersion of lipids by detergents and bile salts
- Breakdown of fat by lipases
- Lipoproteins as lipid carriers (example Chylomicrons)
- Oxidation of fatty acids
- Ketogenesis
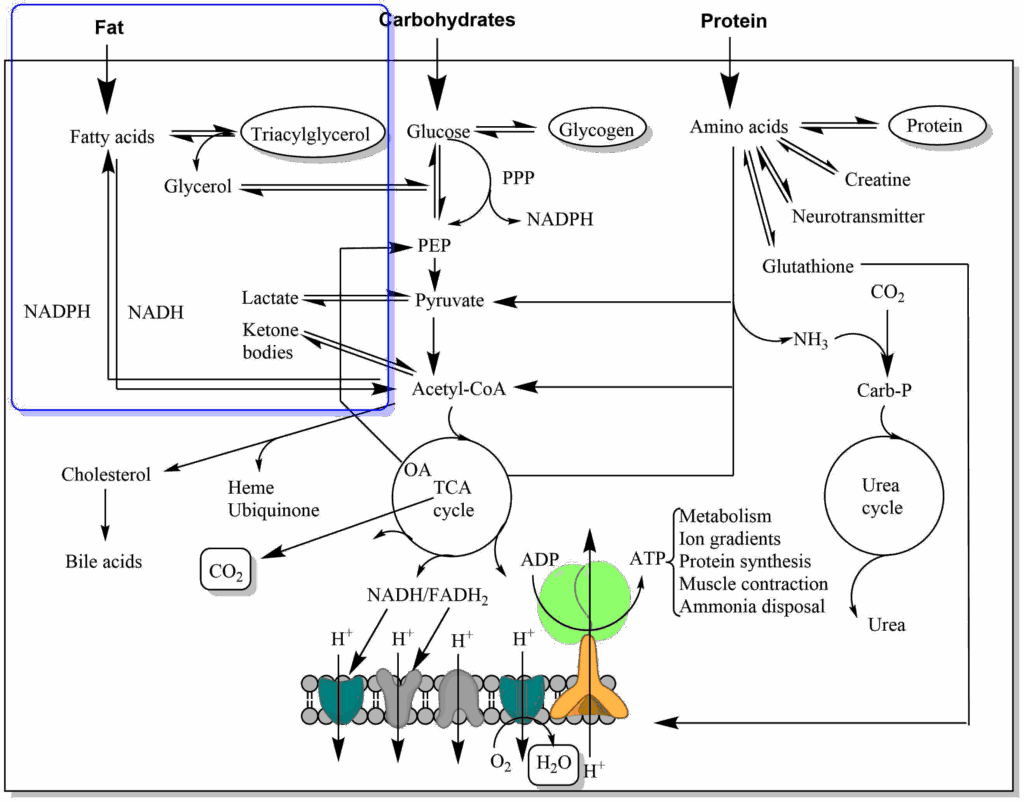
You are now familiar with the digestion and breakdown of carbohydrates. You saw how oxidation of nutrients is used to generate CO2 and that electrons are stored in electron carriers, which are finally used to generate ATP in the respiratory chain. It will now be easier to understand what will happen to other nutrients such as fat and protein. We will have to look for hydrolysis to break down complex molecules into individual building blocks, followed by breakdown of building blocks through oxidation and storage of electrons. We also understand that fat digestion has to end in the TCA cycle to generate CO2. In the case of fat the intermediate molecule joining the pathway we already know is acetyl-CoA, while glycerol enters glycolysis (Fig. 1).
We will now follow the path of fat after food intake. Before we do so let us have a look again what kind of lipids our body needs and what kind of lipids we may find in our nutrition (Fig. 2).
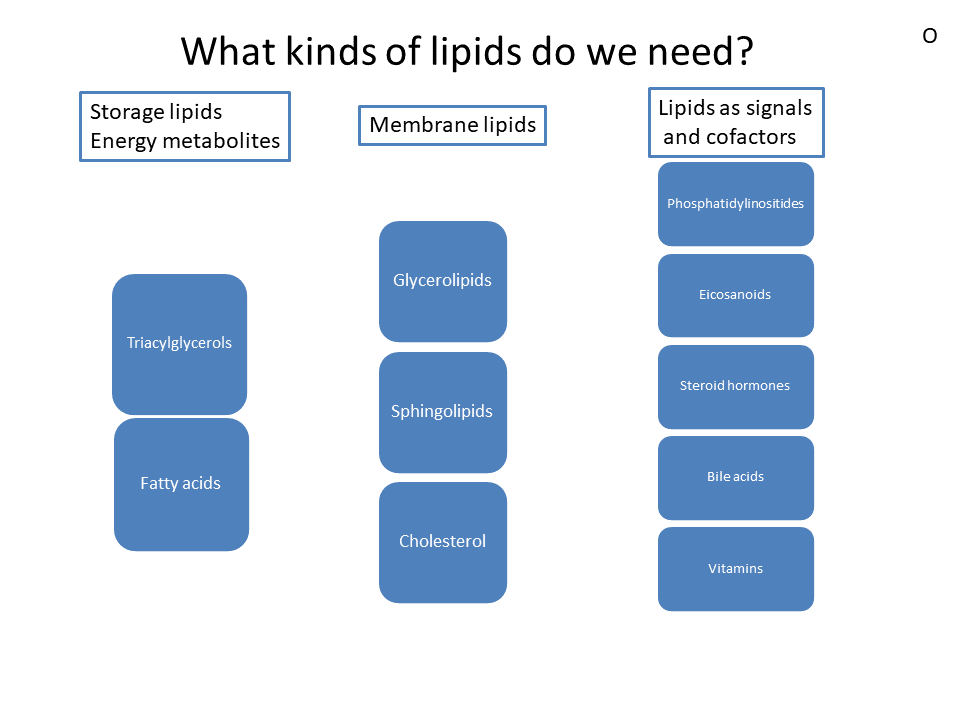
The main nutrient we know as fat is more precisely called triacylglycerol. Fat is a compound metabolite made up of glycerol, which forms an ester with 3 fatty acids (Fig. 3). The average sum formula of fat is C55H104O6. Fat is very hydrophobic and therefore does not mix with water. Membrane lipids form a smaller part of our nutritional lipid intake, see chapter 1 for an overview of the different types of membrane lipids. Glycerolipids and sphingolipids look similar to fat, but one of the fatty acids is replaced by a phospho-organic head-group facing the aqueous environment. Cholesterol is an essential part of membranes and and plays an important role in cardiovascular diseases. We will cover cholesterol in detail in chapter 13. Lipids also play an important role as cofactors in digestion (bile acids), capturing oxygen radicals (vitamin E), as hormones (steroid hormones) and signalling molecules (eicosanoiods and phosphatidylinositides).
Here we will focus on lipids as nutrients with a focus on fat, the most abundant nutrient lipid.
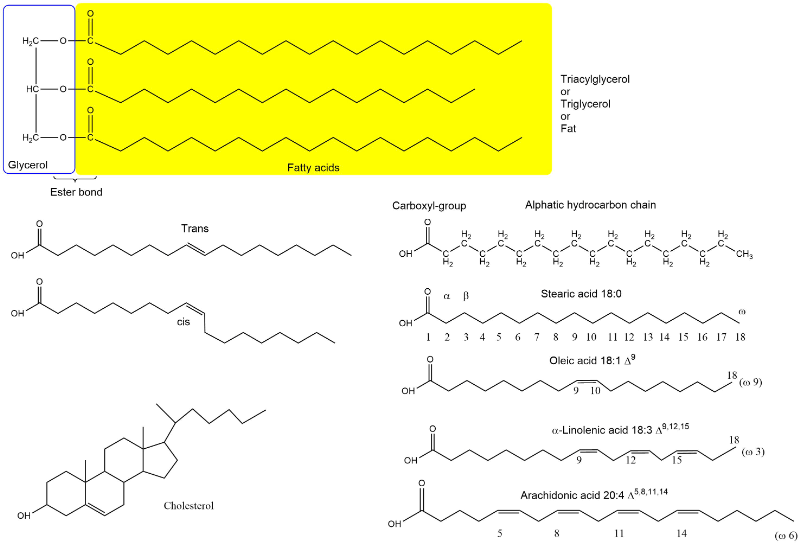
To name the molecules properly and to understand the chemical reactions during breakdown of fats we need to have a look at some nomenclature. The bond between glycerol and fatty acids is an ester bond. An ester bond can also be formed between cholesterol and a fatty acid generating cholesteryl-esters. Fatty acids can have different length, typically 16-20 carbon atoms. In biological systems fatty acids often have double bonds. These are always in cis-configuration. Trans fatty acids are generated in industrial processes, such as hardening of fats and oils to form spreadable toppings. Epidemiological studies have shown that trans-fatty acids promote atherosclerosis and raise LDL levels (see chapter 13). Since then, industrial fat and oil processing has been optimised to prevent generation of trans fatty acids. The carbon atom after the carboxyl-group in fatty acids is the alpha-carbon or C2, carbon atom 3 is the beta-carbon. The main pathway of fatty acid metabolism is called beta-oxidation, because the carbon in beta-position (carbon 3) is oxidised in the process. Our cells can form a double bond at position 9, but they cannot form additional double bonds at position 12 and 15. As a result linoleic acid (double bonds at position 9 and 12) and alpha-linolenic acid (double bonds at positions 9, 12 and 15) (Fig. 3) cannot be formed in humans and are thus termed nutritionally essential fatty acids. Because double bonds cannot be introduced beyond carbon atom 9, the essential fatty acids are often referred to as omega-3 and omega-6 fatty acids, in which the carbon atoms are counted from the end to the first double bond. Omega-3 unsaturated fatty acids are particularly prevalent in fish oil and epidemiological studies suggest that consumption of these fatty acids prevents the onset of atherosclerosis. Polyunsaturated fats are particularly prevalent in oil, fish and nuts. Fat composition is closely linked to the physical properties of fat. Liquid oils are high in unsaturated fat, while solid fat like butter and coconut fat are high in saturated fat (Fig. 4).
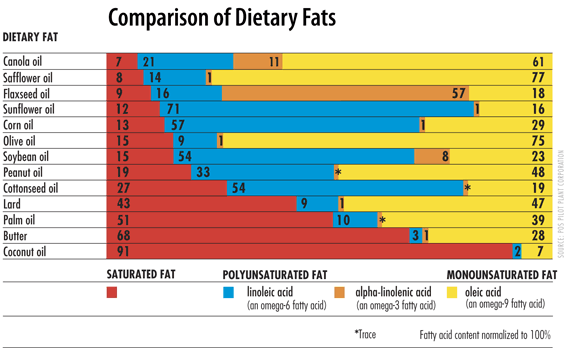
Why are omega-3 and -6 fatty acids essential? We need them to synthesise arachidonic acid, which is a precursor for prostaglandins, thromboxans and leukotriens. These are hormone-like substances that act at a short distance. They are involved in induction of labor, inflammation, blood pressure, pain and platelet aggregation. Moreover, linoleic acid forms a wax-like compound that improves the water impermeability of skin.
As we have done for other metabolites we want to understand the digestion process of fat and lipids. We use fat (triacylglycerol) as an example. An important consideration is the hydrophobicity of fat. Daily experience shows that grease and oil do not dissolve in water but rather generate a solution with oily droplets.

Detergents and bile salts. We know from daily experience that detergents can help dissolve fat. Detergents look similar to membrane lipids (Fig. 5) but are far more soluble in water.
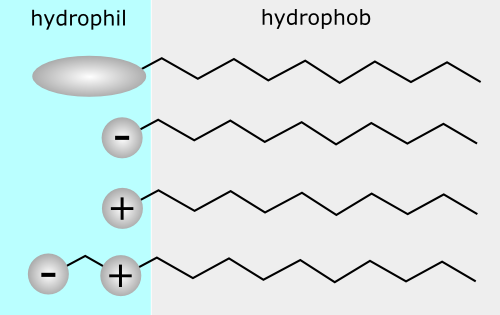
They have a hydrophilic head group and a non-polar hydrocarbon tail, which is much shorter than in fats. The hydrophilic head can be neutral (i.e. a carbohydrate), negatively charged, positively charged or both. One of the most well-known detergents is SDS (sodium dodecylsulfate). What happens if you add a detergent to water?
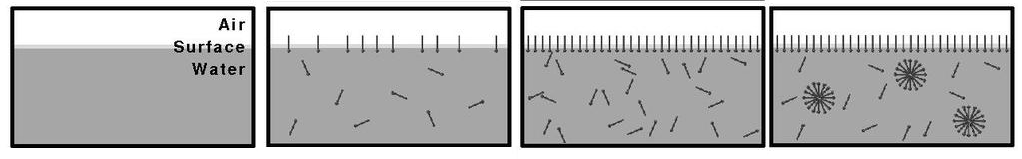
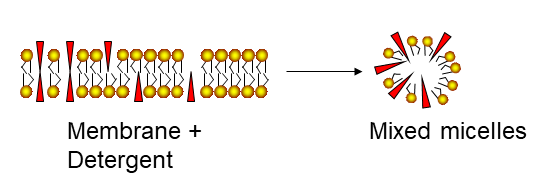
The detergent molecules will initially orient the headgroup towards the water phase and the hydrophobic tail is oriented to the only non-polar phase, the air (Fig. 6). Small amounts can dissolve in water. Upon increasing the concentration, micelles will be formed, which are spheres made up of detergent molecules with head-groups pointed towards the aqueous phase. The micelles are in equilibrium with individual detergent molecules dissolved in water. Membrane lipids and fat are practically insoluble in water. Fat will just float as a mass of fat, while membrane lipids will form membrane bilayers and vesicles. When detergents come into contact with membranes or fat, they can wedge between lipid or fat molecules. Because the polar and in particular charged head-groups repel each other, this will dissolve the membrane and result in the formation of mixed micelles (Fig. 7).
Bile acids are the detergents in our body helping with lipid digestion (Fig. 8). They have a polar and hydrophobic part and thus are mild detergents that can reduce the size of lipid droplets. They are derived from cholesterol.
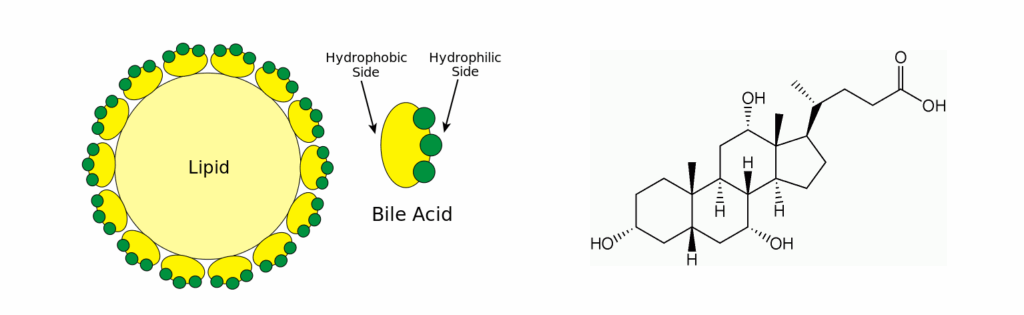
Now that we understand the role of bile acids we can also understand how fat is broken down in the intestine. Lipase is secreted by the pancreas and combines with colipase. Colipase has a hydrophobic face and a hydrophilic face. This allows colipase to bind to bile-acid coated fat droplets (Fig. 9). Lipase can then interact with individual fat molecules and can digest them into two molecules of fatty acid and a molecule of 2-monoacylglycerol.
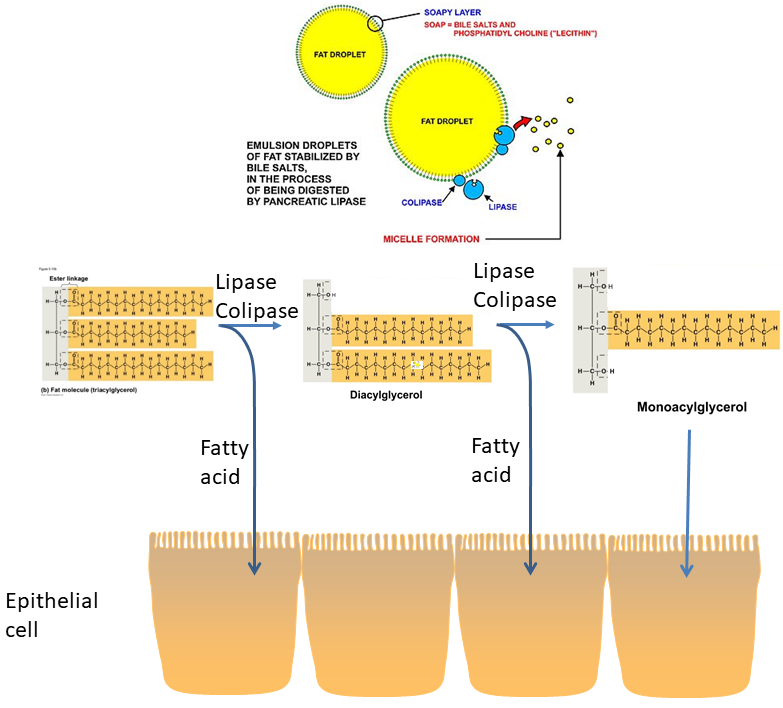
Fatty acids and 2-monoacylglycerol can be taken up by epithelial cells. It appears that a variety of mechanisms are involved in mediating the uptake of fat digestion products into enterocytes, including passive diffusion (long fatty acids), endocytosis and transporter-mediated mechanisms. Once inside the enterocyte, fat is resynthesized and packed into lipid particles called chylomicrons (Fig. 10).
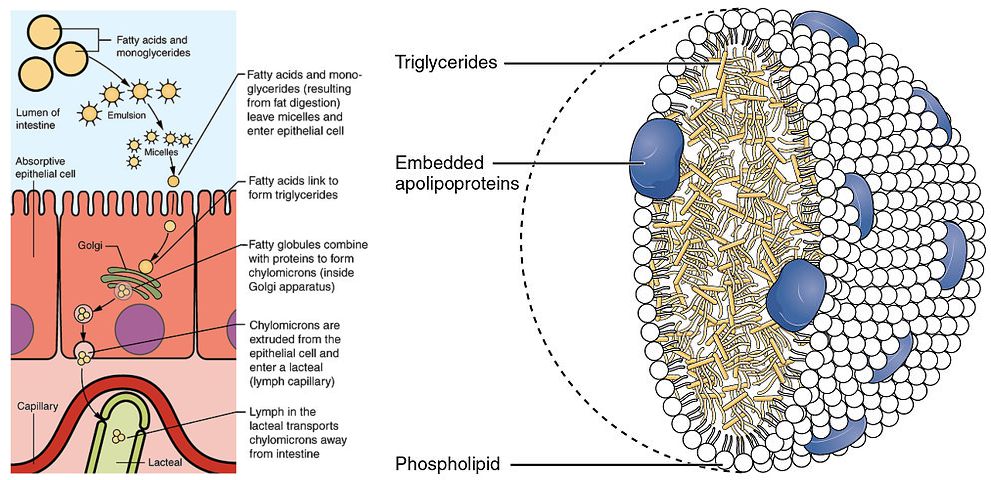
Chylomicrons belong to a larger group of lipoproteins, which transport lipids in the blood stream. We will revisit these particles in chapter 13. These particles are enclosed by a monolayer of phospholipids. The core is filled with triglycerides and cholesteryl esters. Apolipoproteins are embedded in the phospholipid layer. These function as address labels and ensure that the particles will attach to the appropriate enzymes and receptors, which either breakdown and withdraw the contents or engulf the complete particle, respectively. Due to the size of the chylomicrons, they are released into the lymphatic system (lacteal in Fig. 10), not into the blood stream like carbohydrates and amino acids. However, the lymphatic system merges with the circulatory system, thereby being transported to all tissues.
What happens to chylomicrons in the circulatory system? Chylomicrons are too big to fit between the gaps of endothelial cells lining all blood vessels. Instead, chylomicrons bind to lipoprotein lipase located at the surface of endothelial cells (Fig. 11). Lipoprotein lipase then releases fatty acids and glycerol, which can diffuse through the gaps.
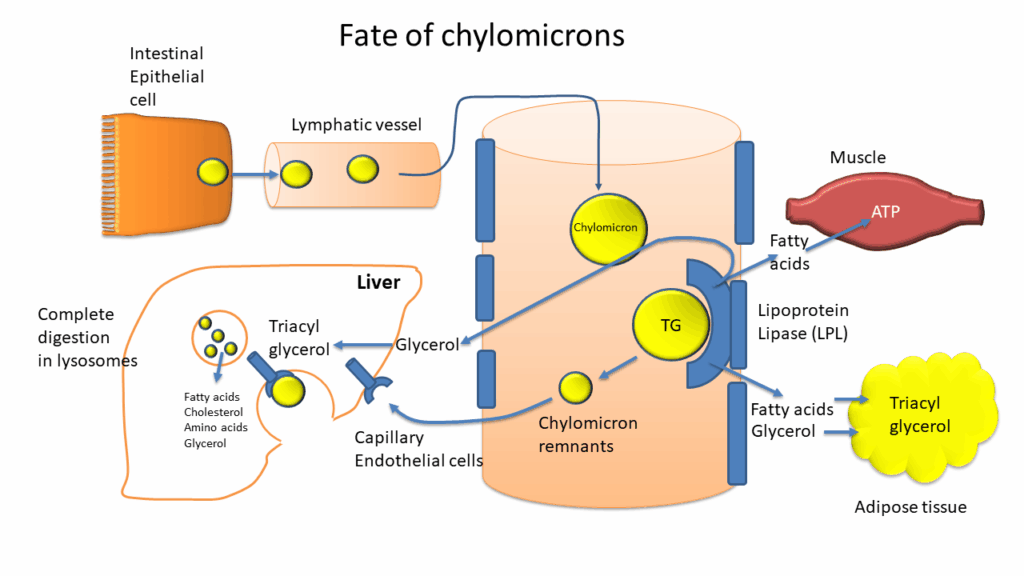
Fatty acids are energy-rich nutrients, which are a preferred energy source for muscle and heart, but, with the exception of the brain, can be used by any tissue to some extent. Glycerol is primarily used by adipose tissue for triglyceride synthesis or by liver for gluconeogenesis. Removal of contents from chylomicrons generates chylomicron remnants, which are removed by the liver through receptor-mediated endocytosis (Fig. 11). We will now follow the fatty acids. The pathway by which fatty acids are initially oxidised is the beta-oxidation pathway (Fig. 12). The name points to chemical reactions occurring at the beta-atom of the fatty acid.
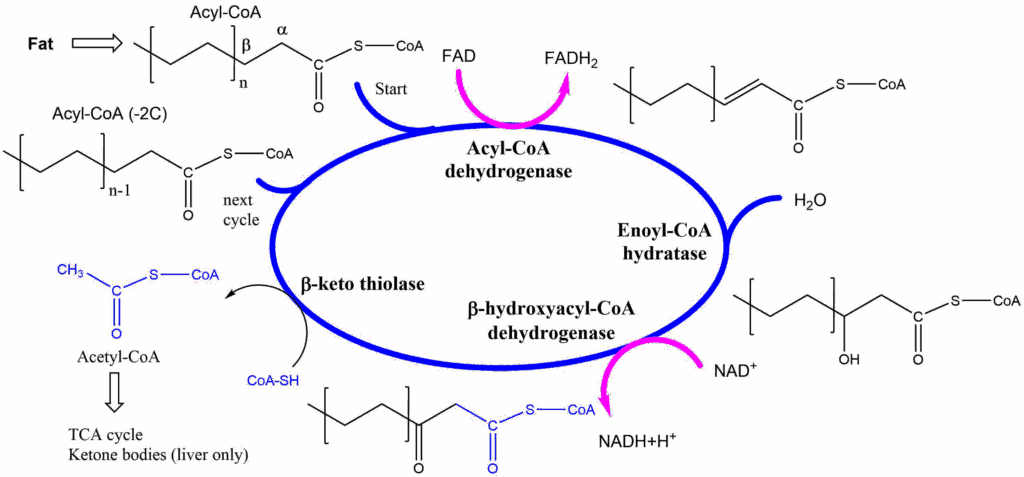
The beta oxidation starts with a thioester of a fatty acid (chapter 3). Recall that thioesters are more chemically reactive than oxoesters, particularly at the beta-atom. The first step of beta oxidation is the removal of two electrons using FAD to form a double bond. Water is added to the double bond resulting in a beta-hydroxy-group. The molecule is oxidised again to form a beta-keto group. Using a molecule of Coenzyme A, the molecule can now be split (thiolysed) to form acetyl-CoA and a fatty acyl-CoA that is 2 -carbon units shorter than the original molecule (n-1 in Fig. 12). The process is continued until the whole fatty acid is broken down into acetyl-CoA units. Odd-numbered fatty acids result in propionyl-CoA in the final step, which is converted to the TCA cycle intermediate succinyl-CoA. You can see clearly that all products of the cycle require further metabolism inside mitochondria (Acetyl-CoA, FADH2, NADH). Not surprisingly, all reactions of the beta-oxidation occur inside mitochondria.
We have not yet explained how a fatty acid becomes activated to fatty acyl-CoA and how it enters into mitochondria. We have seen earlier that antiporters are used to bring metabolites in and out of mitochondria. However, Coenzyme A is a very large molecule and together with a fatty acid difficult to transport across membranes. A smaller carrier, called carnitine is used instead. The molecule is very hydrophilic to provide better accessibility to the transporter. The pathway is called the carnitine shuttle (Fig. 13).
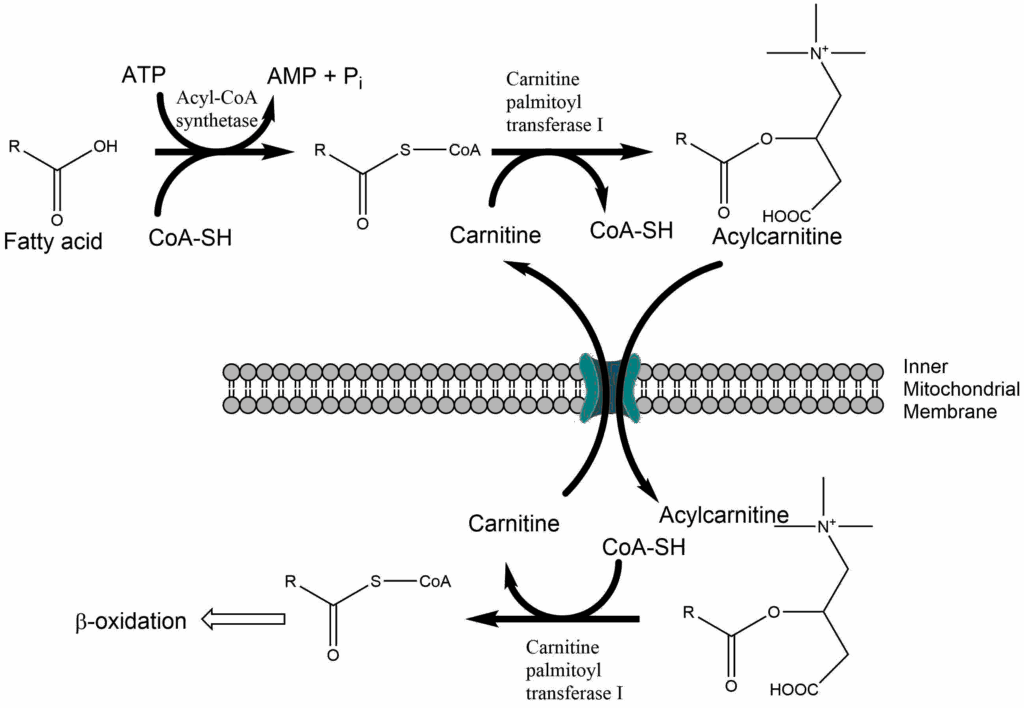
First fatty acids are activated using ATP to generate a chemically activated fatty acyl-CoA. This reaction could be considered the activation phase of beta-oxidation, similar to the activation phase of glycolysis. Carnitine palmitoyl transferase I (CPTI) is located in the outer mitochondrial membrane and catalyses the condensation of carnitine with the fatty acid while CoA-SH is released. Acylcarnitine is transferred into the mitochondria in exchange for carnitine. In the inner mitochondrial membrane CPTII is located, which releases carnitine in exchange for CoA-SH. Thus chemically activated acyl-CoA is reformed within the mitochondria. Please note that the outer mitochondrial membrane contains large pores, which allow the passage of fatty acids, CoA-SH and ATP etc.
Carnitine deficiency is a genetic condition caused by mutations in the transporter that translocates carnitine across the plasma membrane. You learned in this chapter that fatty acids are a preferred energy source for muscle and heart. As a result muscle weakness is observed in this condition. If carnitine deficiency is severe, heart muscle weakness can occur as well. Another inherited disease, carnitine palmitoyl transferase deficiency, also causes muscle weakness. Switching to a diet high in carbohydrates can be helpful to reduce the reliance of muscle and heart on fatty acids.
You can now appreciate the use fat as an energy source, particularly as a fuel for exercise. Fat is far more difficult to use for people with a sedentary lifestyle. We will discuss these problems in later chapters.
The ketogenic diet
The ketogenic diet is rich in fat and low in carbohydrates. What happens when we eat a lot fat? You learned above that beta oxidation generates a large amount of acetyl-CoA. Eating a diet rich in fat induces a metabolic program in the liver called ketogenesis or synthesis of ketone bodies (Fig. 14). The name ketone bodies is derived from the generation of acetone as a spontaneous byproduct. This generates a typical aromatic smell of the breath and can be detected by a breathalyzer (see video below). In liver mitochondria excess amounts of acetyl-CoA derived from fat are used to generate 3-Hydroxy-3-methyl-glutaryl-CoA. This is a precursor for ketone bodies and cholesterol. Removal of acetyl-CoA generates acetoacetate, which can be reduced to beta-hydroxybutyrate. Although chemically not a “ketone”, beta-hydroxybutyrate is considered a ketone body, because of its physiological role. Acetoacetate and beta-hydroxybutyrate are released by the liver and are taken up by extrahepatic tissues for energy generation. You can consider ketone bodies a transportable form of acetyl-CoA with liver being the source and other organs the sink. The breakdown in extrahepatic tissue is only a partial reverse of the synthesis. Oxidation generates acetoacetate and yields NADH. Acetoacetate is converted to acetoacetyl-CoA and subsequently thiolysed to form two molecules of acetyl-CoA.
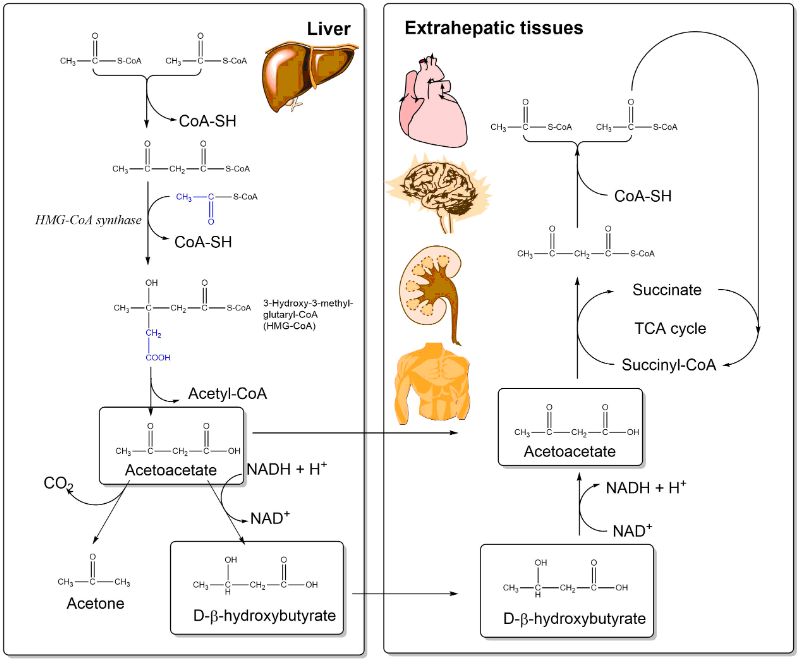
You saw in chapter 9 that acetyl-CoA is an inhibitor of the pyruvate dehydrogenase reaction. Thus an excess of fat will result in reduced metabolism of glucose. This is one of the effects of the ketogenic diet. Another effect of the ketogenic diet is enhanced synthesis of cholesterol, which in turn can lead to atherosclerosis. Physiologically, ketone body biosynthesis is a metabolic program associated with long-term starvation. Long-term starvation will mobilise fat reserves, which are converted to ketone bodies in the liver. The transfer to other organs is a mechanism to provide extrahepatic organs with a nutrient that reduces the reliance on glucose. In individuals who have type I diabetes (juvenile diabetes), peripheral organs cannot use glucose very well. This is similar to starvation and induces ketogenesis in type I diabetes. The smell of acetone is typical for poorly controlled diabetes.
Watch the video to see the effects of ketone body production in your body.
Alcohol
A “nutrient” closely related to fatty acids is ethanol. Its metabolism initially generates acetaldehyde, which is converted into acetate mostly in the liver and subsequently released. As a fatty acid, acetate is primarily used by muscle and heart (Fig. 15).
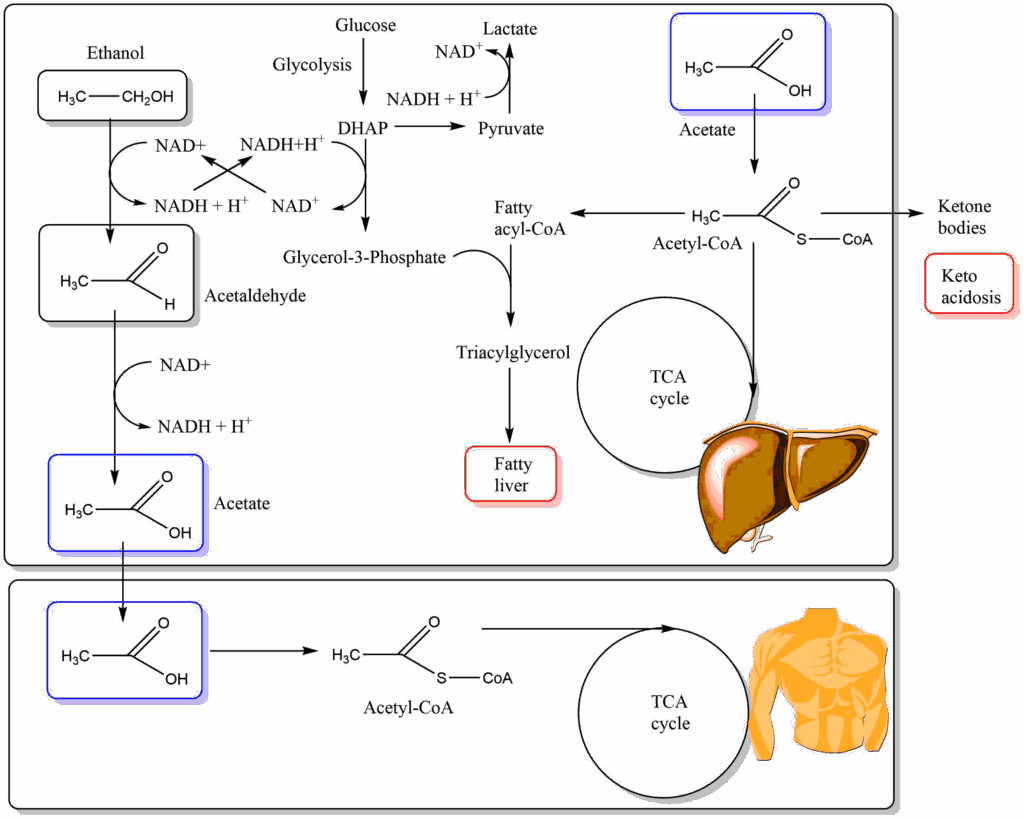
Metabolism of ethanol generates NADH in the liver cytosol (alcohol dehydrogenase) and also inside mitochondria (aldehyde dehydrogenase). As shown in Fig. 15 NADH generated in the cytosol leads to the conversion of glycolysis intermediate dihydroxyacetone-phosphate (DHAP) to glycerol-3-phosphate, which is one of the building blocks to make fat. In addition acetate can be converted to acetyl-CoA also in the liver, which is the second building block to make fat. Thus chronic alcohol intake generates fatty liver. It also acidifies the blood through generation of lactate (pushed by NADH in the cytosol) and ketone bodies. Mitochondrial NADH will cause the TCA cycle to slow down, further pushing the generation of fat and ketone bodies. Elevated levels of NADH will move the pyruvate/lactate and oxaloacetate/malate ratios towards malate and lactate and thereby remove intermediates for gluconeogenesis (pyruvate and malate). Hypoglycemia is a potentially dangerous complication of alcohol addiction.
- Fatty acids are categorised as saturated or unsaturated. The human body cannot synthesize fatty acids with double bonds beyond position 9. Fatty acids with double bonds beyond position 9 are called essential fatty acids (omega-3 and omega-6).
- In fat (triacylglycerol) three fatty acids form esters with glycerol. Fat is not soluble in water.
- Fat digestion requires detergents (bile acids) to break up fat molecules sticking together. This allows binding of colipase to fat droplets, which in turn binds lipase. Pancreatic lipase breaks down fat into two molecules of fatty acid and a molecule of 2-monoacylglycerol.
- Fatty acids and 2-monoacylglycerol are transported into enterocytes by a variety of mechanisms. Inside enterocytes fat is resynthesised and assembled together with phospholipids and cholesterylesters into chylomicrons. Chylomicrons pass into the lymphatic system and then into the blood stream.
- The content of chylomicrons can be released by lipoprotein lipase, which is found in endothelial cells lining blood vessels. This results in the generation of fatty acids and glycerol, which can diffuse from the blood vessel to tissue cells. Most of the glycerol is used by adipose tissue to form fat for storage or by liver to generate glucose.
- Fatty acids can be used by many cells to generate energy after conversion to fatty acyl-CoA. They are metabolised through beta-oxidation, a cyclic sequence of reactions which generate acetyl-CoA from each two subsequent carbon atoms. In the process FADH2 and NADH is generated. Further energy is generated by the complete oxidation of acetyl-CoA in the TCA cycle.
- Beta-oxidation occurs in the mitochondrial matrix. Fatty acids enter the mitochondria via the carnitine shuttle.
- The ketogenic diet is a fat-rich, carbohydrate-low diet that induces ketone body synthesis in the liver. Ketone bodies are released from the liver and used by extrahepatic tissues to generate acetyl-CoA.
- Alcohol is oxidised into acetate in a two-step process in the liver. Acetate is then converted into acetyl-CoA in many tissues to generate energy or to synthesise fat. The NADH generated by alcohol dehydrogenase in the cytosol leads to synthesis of fat and ketone bodies.
The image shows two blood samples after centrifugation, explain the difference.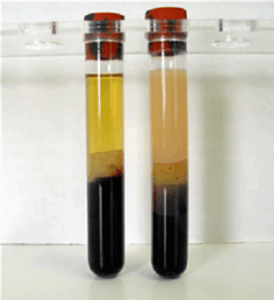
The right sample shows blood after a fatty meal. Chylomicrons generate a milky suspension. The sample on the left is from a fasting person. It is clear.
Compare the reactions of beta oxidation with those of the TCA cycle.
- Which of the reactions below is similar to Enoyl-CoA Hydratase?
- Which of the reactions below is similar to Acyl-CoA Dehydrogenase?
- Which of the reactions below is similar to beta-hydroxyacyl-CoA dehydrogenase?
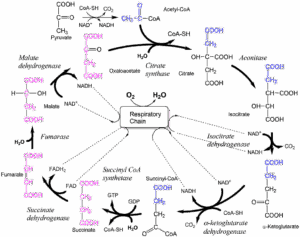
- Fumarase
- Succinate dehydrogenase
- Malate dehydrogenase
Calculate the number of ATP molecules generated by a mol of palmitic acid (saturated fatty acid C16). Use the approximation of 2.5 moles of ATP generated for each NADH and 1.5 moles of ATP for each FADH2. Take into account activation of palmitic acid and what happens in the final cycle.
Oxidation of palmitic acid yields 7 NADH + 7 FADH2 + 8 acetyl-CoA in 7 cycles of mitochondrial beta oxidation. Every acetyl-CoA yields 3 NADH + 1 FADH2 + 1 GTP (=ATP) during Krebs cycle. Considering an average production of 2.5 ATP/NADH and 1.5 ATP/FADH2 using the respiratory chain, you have 108 ATP molecules (3×2.5+1×1.5+1=10; 10×8 =80 from acetyl-CoA, 7×2.5+7×1.5=17.5+10.5=28 from beta-oxidation, total 108). However, 2 ATP molecules were consumed during the initial activation of Palmitate to Palmitoyl-CoA which is going to be oxidized in the mitochondria. So, net energy output = (108 – 2) = 106 ATP.
Dying muscle cells would spill their contents into the blood. Occurrence of these enzymes suggests that muscle tissue is dying.
What are the major fuel for muscle cells?
Muscle can use both glucose and fatty acids, but prefers fatty acids.
Why might plasma levels of fatty acids be elevated?
If fatty acids are not metabolized by muscle, less fatty acids would be removed from the blood resulting in increased plasma fatty acid concentrations, particularly after exercise or a meal.
Why might blood glucose levels be low, particularly after exercise?
Since fatty acids cannot be metabolized by muscle cells, glucose is used at a higher rate.
Chronic alcohol intake can cause inflammation of the pancreas resulting in reduced secretion of digestive enzymes. A patient with acute pancreatitis eats a full diet and his/her stools became bulky, glistening, yellow-brown and foul-smelling. They floated on the surface of the toilet water. What caused this problem.
The reduced secretion of lipases caused malabsorption of dietary fats. The fats remained in the digestive tract and caused the changes to the stool.
Match the elements of lipid digestion with their corresponding function.
- Bile acids
- Colipase
- Carnitine
- Lipoprotein lipase
- beta-oxidation
A) Metabolic pathway that breaks down fatty acids.
B) Disperse fat and lipids by forming mixed micelles
C) Hydrolyses triacylglycerols transported in chylomicrons
D) Binds to fat droplets and activates lipase
E) Forms esters with fatty acids to facilitate transport into mitochondria
1 – B
2 – D
3 – E
4 – C
5 – A
What is the difference between the type of oxidation catalysed by acyl-CoA dehydrogenase and that catalysed by beta-hydroxyacyl-CoA dehydrogenase?
Acyl-CoA dehydrogenase removes electrons from adjacent atoms creating a double bond and using FAD as a coenzyme. Beta-hydroxyacyl-CoA dehydrogenase removes two electrons from a single carbon atom thereby oxidising an alcohol to a ketone. It uses NAD as a cofactor.
- Fig. 1 By the author using ChemDraw
- Fig. 2 By the author using Powerpoint
- Fig. 3 By the author using ChemDraw
- Fig. 4: Vwalvekar [GFDL (http://www.gnu.org/copyleft/fdl.html)], via Wikimedia Commons
- Fig. 5: Roland.chem [GFDL (http://www.gnu.org/copyleft/fdl.html) or CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/)]
- Fig. 6 Schmin [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], from Wikimedia Commons
- Fig. 7 By the author using Powerpoint
- Fig. 8 Bile1.png: Frank Boumphrey, MDderivative work: Hazmat2 [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons
- Fig. 9 By the author using Powerpoint and incorporating Cruithne9 [CC BY-SA 4.0 (https://creativecommons.org/licenses/by-sa/4.0)], from Wikimedia Commons modified by the author
- Fig. 10: OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons and
- Fig. 11 By the author using Powerpoint
- Fig. 12 By the author using ChemDraw
- Fig. 13 By the author using ChemDraw
- Fig. 14 By the author using ChemDraw
- Fig. 15 By the author using ChemDraw