Synthesis of Fatty Acids and Lipids
- Understand how fatty acids and fat are synthesised.
- Understand how fat is distributed from the liver to all organs.
- How fat is mobilised when required to generate energy.
- Insulin resistance, lipotoxicity
- Conversion of sugar into fat
- Enzyme regulation by protein phosphorylation
- Fatty acid biosynthesis
- Signal transduction cascade, second messenger
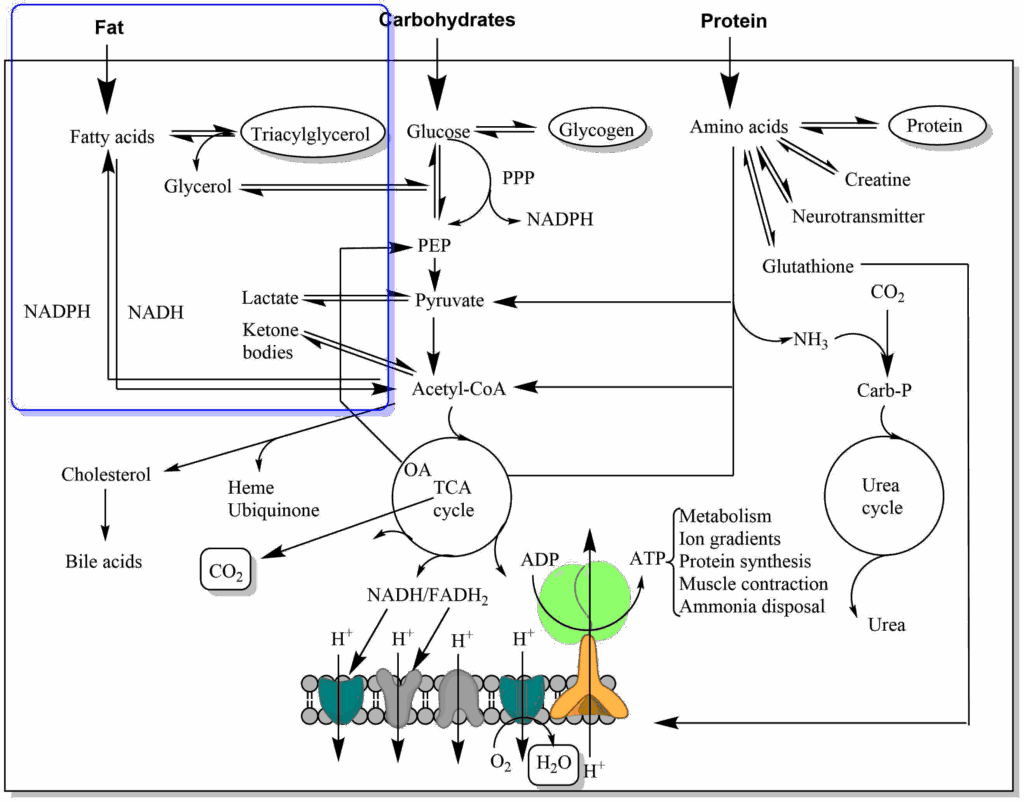
Fat is the most important energy storage in our body. Even in a slim individual it can provide energy for many days. The advantage of storing fat over storing carbohydrates or protein is that fat stores do not bind additional water, whereas hydrophilic molecules like protein and carbohydrates are highly hydrated, adding weight. In contrast to glycogen, which has an upper limit of storage, fat can be stored in unlimited amounts leading to increasing obesity in the general population.
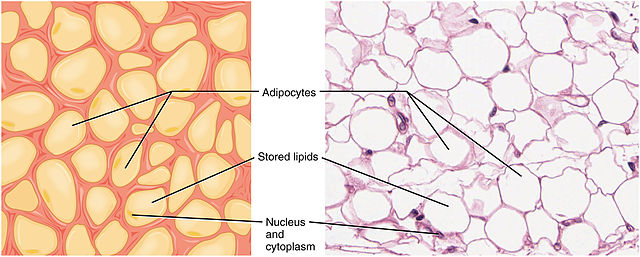
The inside of adipocytes is almost entirely filled up with fat, the nucleus and aqueous cytoplasm being pushed to the fringes (Fig. 1). Initially it was thought that adipocytes are passive storage cells, but it is now recognised that adipose tissue releases a number of hormones to regulate appetite and body metabolism.
In Chapter 11, we followed the fate of fat as a nutrient and saw that it can be used to generate energy through beta oxidation. However, much of the nutrients we eat are in excess of acute energy requirements and are stored as polymers (carbohydrates and amino acids) or aggregates (fat). We saw that fatty acids and glycerol are released from chylomicrons by lipoprotein lipase. Thus fat is not brought to adipose tissue as fat, it is brought to adipose tissue as glycerol and fatty acids. In addition, glucose is taken up by adipose tissue after a meal.
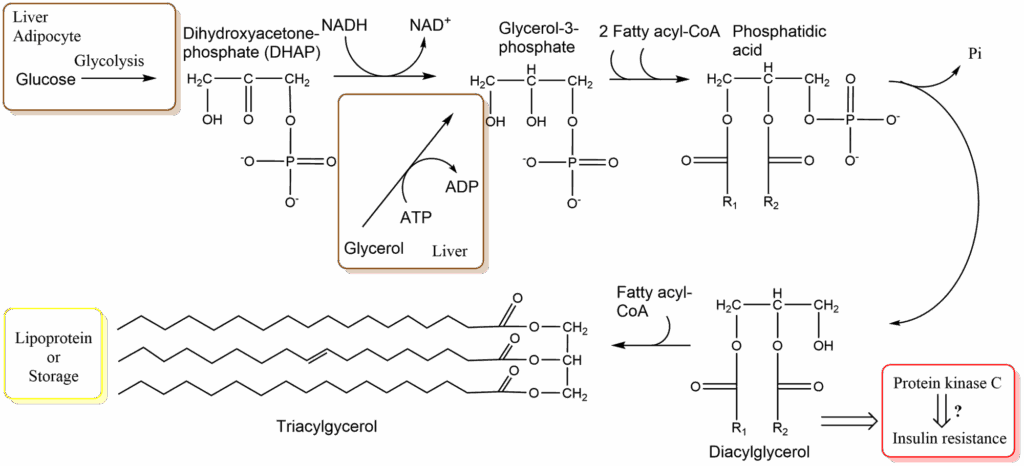
Liver and adipocytes can synthesise fat using glycerol-3-phosphate and fatty-acyl-CoA (Fig. 2). Fatty acyl-CoA is formed in an ATP-consuming reaction between a fatty acid and coenzyme A (see chapter 3, Fig. 7). Glycerol, which is released by lipoprotein lipase, can return to the liver and be used for the reformation of fat. Phosphatidic acid occurs as an intermediate in the pathway. After hydrolysis of the phosphate diacylglycerol is generated, which can react with another fatty acyl-CoA to form fat.
Lipotoxicity is a widely used term referring to the unhealthy effects of fat over consumption. But what does it actually mean? Triacylglycerol is chemically inert and due to its hydrophobic nature does not interact much with other components of the cell. However, diacylglycerol (DAG) occurs as an intermediate of fat synthesis. DAG is an activator of protein kinase C, which in turn phosphorylates components of the insulin signalling network. This is known to reduce the responsiveness of tissues to insulin, a state called insulin resistance. Although highly disputed (therefore “?” in Fig. 2), one theory of lipotoxicity is that elevated levels of DAG occur in individuals that synthesise large amounts of fat and this causes insulin resistance, which leads to the development of type 2 diabetes.
Another theory suggests that elevated levels of ceramide cause insulin resistance and type 2 diabetes. This membrane lipid is synthesized starting from serine and palmitoyl-CoA, two products which are abundant when nutrient intake is elevated (Fig. 3).
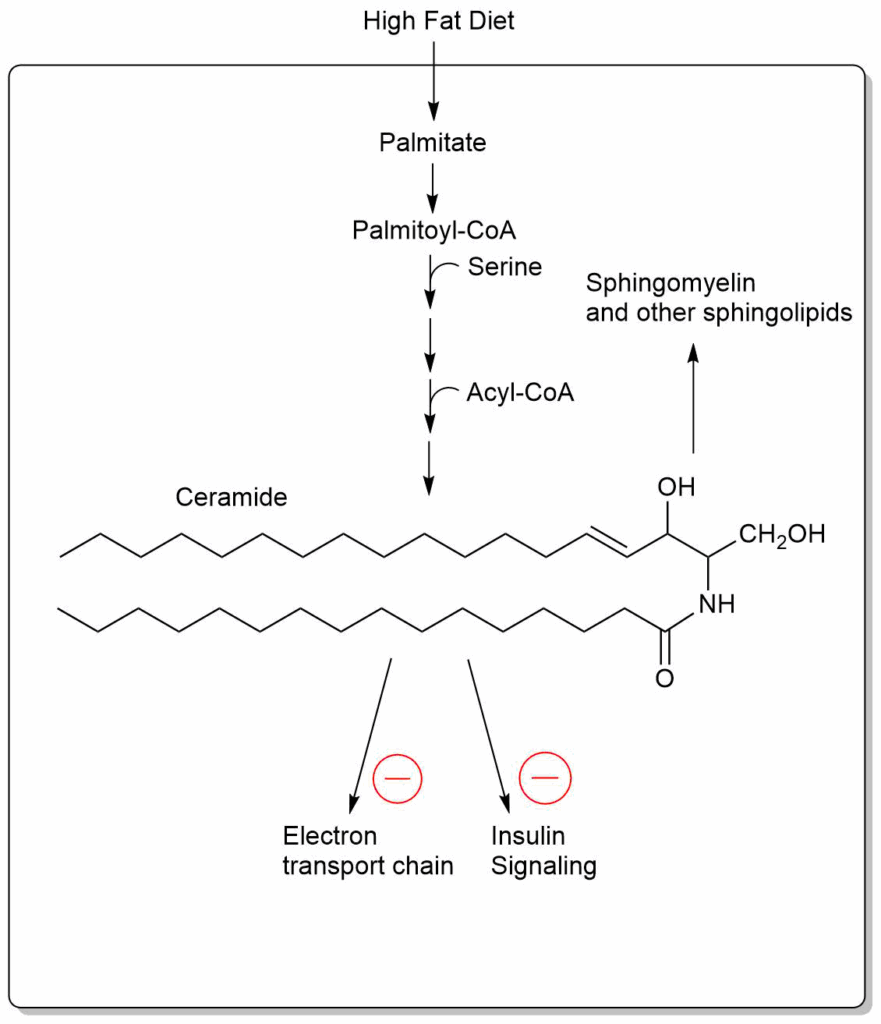
Elevated levels of ceramide inhibit the respiratory chain and reduce insulin signalling.
Please go back to chapter 10 “Real world application” to review another controversial theory of lipotoxicity.
From our own experience we know that consumption of large amounts of carbohydrates (pasta , sugar etc.) can make us fat. The conversion of sugar into fat actually occurs in the liver, in adipose tissue glucose is largely used to generate glycerol-3-phosphate. Thus we need to understand how fat can be generated from sugar. An overview is shown in Fig. 4.
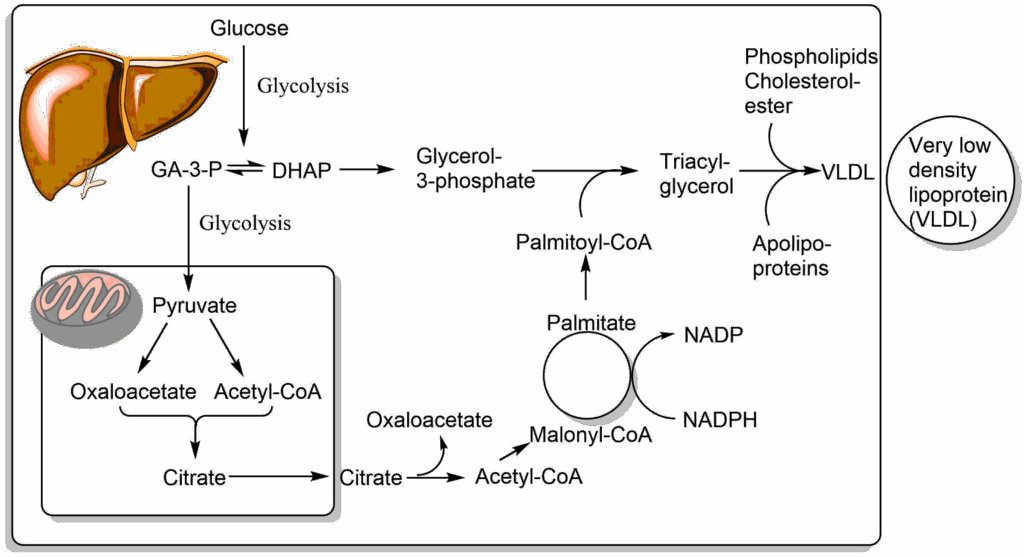
Like in adipose tissue, glucose is used to generate glycerol-3-phosphate. However, fatty acids are synthesised from acetyl-CoA, which ultimately is derived from glucose, as well. It is important to note that the synthesis of fatty acids and fat occurs in the cytosol, while acetyl-CoA is generated inside mitochondria. Acetyl-CoA cannot be transported across membranes, but citrate, which is formed from acetyl-CoA and oxaloacetate, can cross the mitochondrial membrane using the citrate carrier. Once in the cytosol the reverse reaction occurs, facilitated with the help of ATP, and acetyl-CoA is reformed. Acetyl-CoA is then converted into malonyl-CoA, which is the building block to generate fatty acids (more details below), which are typically 18 carbon atoms long (palmitoyl-CoA). The process of fatty acid synthesis is not simply the reverse of beta-oxidation. Notably, NADPH is used to transfer electrons, the reactions occur in the cytosol and are catalysed by a large complex called fatty acid synthase.

Justus von Liebig (1803-1873) was the first scientist to discover that carbohydrates are converted into fat. He observed that a lean goose weighing 4lbs gained 5 lbs in weight in 36 days during which it was fed with 24 lbs of maize. 3.5 lbs of this weight gain was in the form of fat, which could not have come directly from the maize as it contains less than 0.1% of fat.
In his autobiography Liebig recounted that one of his early teachers characterized him as a “plague of his teachers and a sorrow of his parents” and asked him what he would possibly achieve in the future. He answered that he would become a chemist, which generated uncontrolled laughter of his fellow students. Perhaps for this reason, Liebig was the first academic to introduce practical laboratory classes in universities.
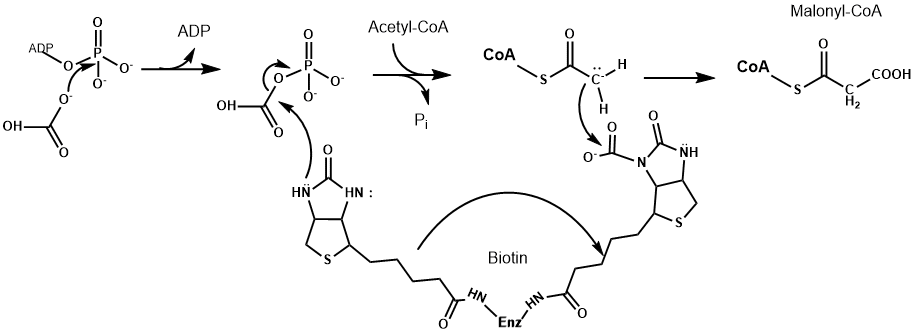
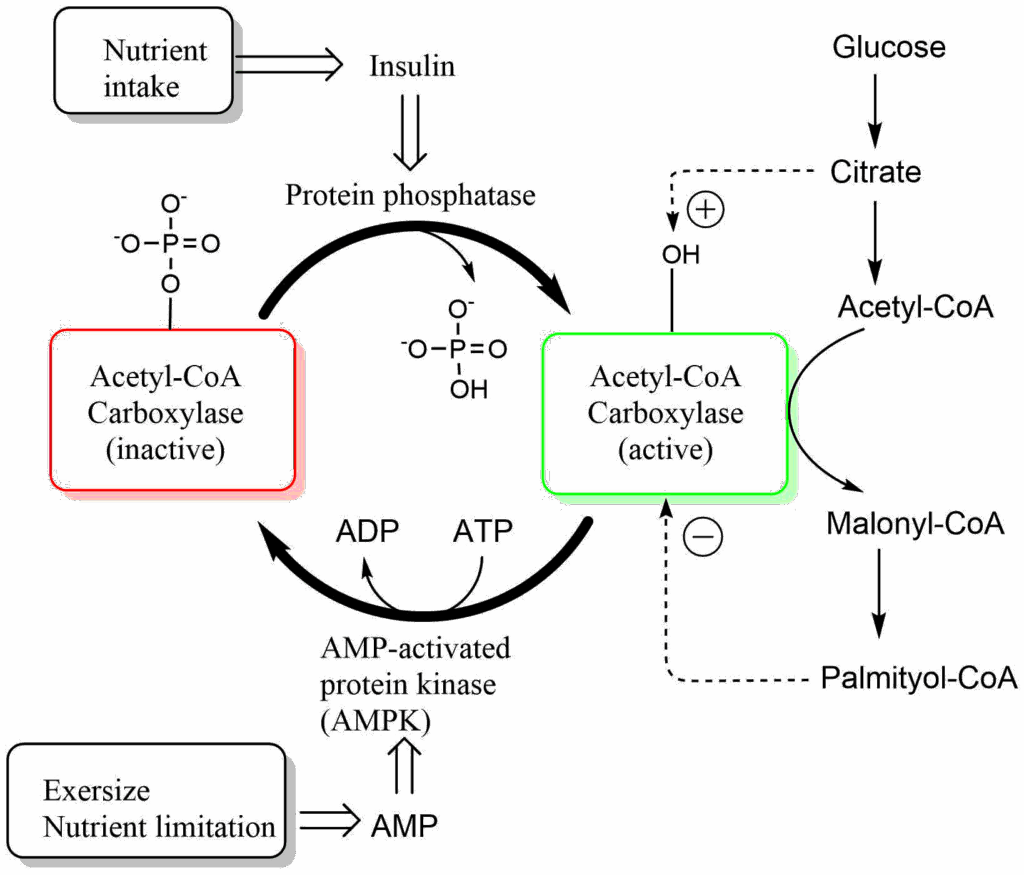
Similar to other enzymes, such as pyruvate dehydrogenase, a vitamin is used as an essential part of the catalytic mechanism, in this case biotin. Biotin is used as carrier of carbon dioxide in all enzymes that carry out carboxylations. Biotin is covalently linked to a lysine residue of the enzyme. ATP is used to generate a reactive form of carbon dioxide, namely carbonyl-phosphate, which is then attached to biotin. Biotin is used to transfer this activated form of carbon dioxide onto acetyl-CoA generating malonyl-CoA. As in the TCA cycle, coenzyme A chemically activates the -CH3 of acetate to react as a carbanion. As we will see below malonyl-CoA is the starter molecule to initiate fatty acid biosynthesis. Not surprisingly, this initiator step is regulated by the nutrient state. After a meal, when insulin is released, the enzyme is activated by dephosphorylation (see detour below for further information on regulation by protein phosphorylation). The active enzyme is in addition regulated by allosteric mechanisms. Elevated levels of citrate further activate the enzyme, while the end-product of fatty acid biosynthesis, palmitoyl-CoA is an allosteric inhibitor. During exercise and nutrient limitation AMP levels are elevated, which activates a protein called AMP-kinase (see chapter 19). AMP-kinase phosphorylates and thereby inactivates acetyl-CoA carboxylase. This ensures that fat is only synthesized when excess amounts of glucose are available.
We have now generated the two precursors for fatty acid biosynthesis in the cytosol, namely acetyl-CoA and malonyl-CoA. Both are brought together on the fatty acid synthesis complex (Fig. 8). Initially acetyl-CoA and malonyl-CoA are attached to a long flexible arm of the fatty acid synthase, called the acyl carrier protein (ACP). It has a phosphopantheteine arm that looks the same as in coenzyme A, but is attached to ACP. The acetyl-moiety and malonyl- transfer from coenzyme A to ACP (1). This arm brings the reactants to the appropriate place on the enzyme. In the case of acetyl-CoA it is transferred to a cysteine residue of the enzyme (2), while malonyl-CoA remains attached to ACP. The two molecules condensate to form acetoacetyl-ACP (3). In the process CO2 is released. Thus malonyl-CoA is used as an activated from of acetyl-CoA.
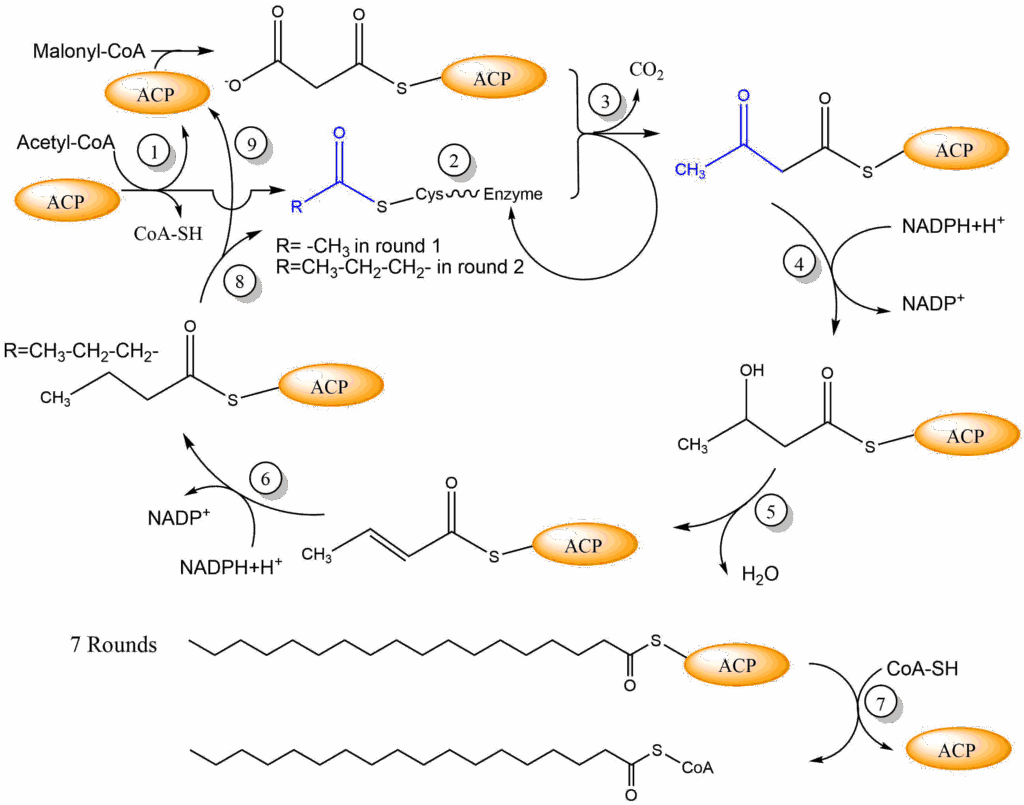
The next reactions resemble the reverse of beta-oxidation, except that NADPH is used as an electron donor. First, the beta keto-group is reduced to a beta-hydroxy group (4). Second, water is eliminated to form a double bond (5), which is then reduced to a single bond using another molecule of NADPH (6). We have now extended the fatty acid by two carbon atoms and reduced the molecule twice to generate an aliphatic fatty acid. The reduced fatty acid is now transferred from ACP to Cysteine-SH (8) to make place for the next incoming malonyl-CoA to be attached to ACP (9). Thus malonyl-CoA is always brought in via ACP, whereas the fatty acyl-CoA (acetyl-CoA in the first round) is brought into the condensation reaction via the cysteine side chain. This cycle occurs seven times until palmitoyl-ACP is formed and is released by thiolysis to form palmitoyl-CoA (7). Watch the video below to appreciate the complexity of fatty acid synthase. The final fatty acyl-CoA can now be used to synthesize fat (Fig. 3).
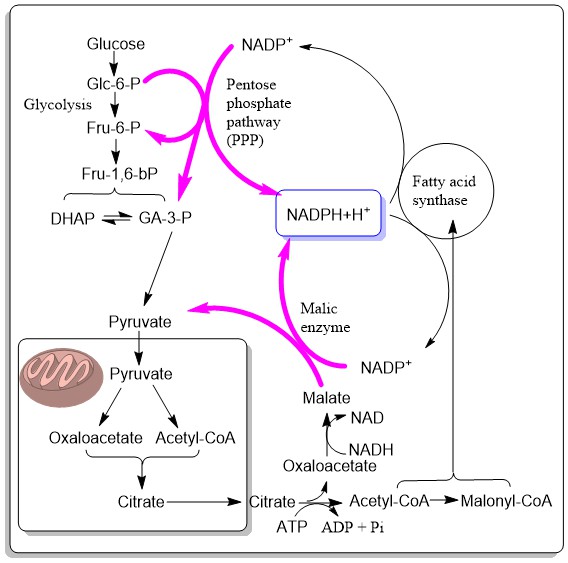
There are a few issues to be resolved before we fully understand the synthesis of fat. The fatty acid synthase uses large amounts of NADPH (2 per cycle). These must be generated in the cytosol. Two pathways are known. One is the pentose phosphate pathway, a bypass of glycolysis, which we will discuss in more detail in chapter 17. The other pathway is the conversion of cytosolic oxaloacetate back into pyruvate via malic enzyme. This pathway recycles the oxaloactetate – generated by citrate lyase in the production of cytosolic acetyl-CoA – to pyruvate, which can go back into mitochondria. The two pathways are illustrated in Fig. 9.
The other question to be answered is the fate of fat produced by the liver. As long as normal amounts of sugar are consumed, these are converted to fat and packed together with phospholipids and cholesterylesters into Very Low Density Lipoprotein (VLDL) (Fig. 4). VLDL is another lipoprotein, like chylomicrons that are used to transport lipids in the blood stream. These are assembled in the endoplasmic reticulum of hepatocytes and released via the Golgi apparatus into the extracellular space. Excessive consumption of sugar and fat will overwhelm this pathway and cause fatty liver disease (Non-alcoholic steatohepatosis [NASH] and Non-alcoholic fatty liver disease [NAFLD]). Fatty liver disease typically develops from NASH to NAFLD and can result in liver fibrosis. Fibrosis is an advanced stage of disease where functional tissue is replaced by non-functional connective tissue, thus compromising liver function.
We have now learned how fat is stored, but how can we mobilise fat when we need it?

We need our fat reserves when we exercise (Fig. 10) or when we are fasting. How is this metabolic program initiated? In this book we frequently look at the changes of metabolic states between feeding and fasting. Fig. 11 recapitulates the synthesis of triglycerides in the fed state and contrasts it to the mobilisation of fat when fasting. Synthesis is driven by the release of fatty acids from chylomicrons (from the intestine, derived from fat in the nutrition) and VLDL (from the liver, fat derived from glucose). Fatty acids are taken up by adipocytes. At the same time glucose uptake is stimulated by insulin and subsequently metabolised to glycerol-3-phosphate. Together they form triacylglycerol, which is deposited as fat droplets.
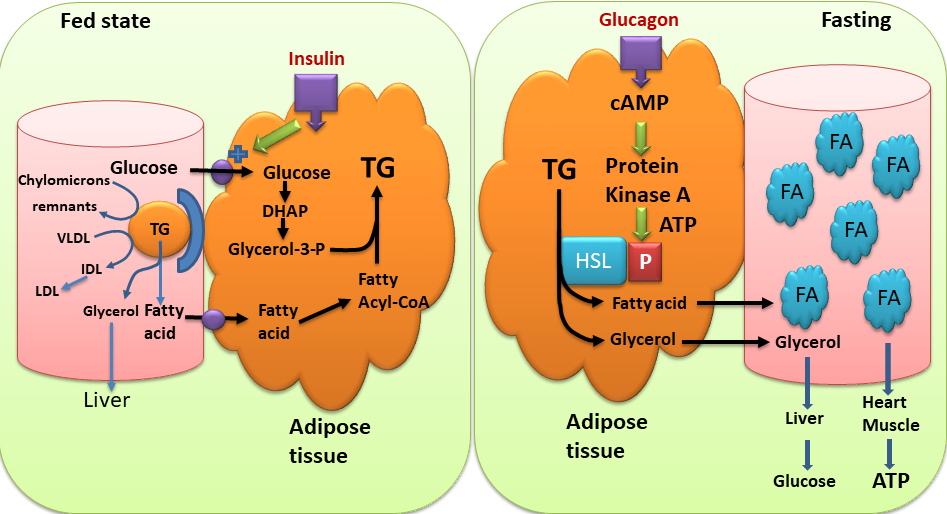
When fasting, glucagon is the dominant hormone (Fig. 11). When glucagon is elevated, insulin is low and vice versa. Glucagon stimulates the hydrolysis of triglycerides into fatty acids and glycerol by hormone-sensitive lipase (HSL). Hormone sensitive lipase becomes activated through phosphorylation (see detour below) by protein kinase A. When fatty acids are released, they bind to serum albumin in the blood stream and get transported to other tissues, such as heart and skeletal muscle, where they are used to generate ATP. Synthesis and breakdown of fat are regulated reciprocally. Malonyl-CoA is a strong allosteric inhibitor of carnitine palmitoyl transferase I (CPT I, chapter 11). Thus, when malonyl-CoA is produced for fatty acid biosynthesis it automatically inhibits beta-oxidation. In fact, there is a specific isoform of acetyl-CoA carboxylase (ACC2), which produces malonyl-CoA just as a regulator of CPT I. Like ACC1 (Fig. 7), ACC2 is regulated by insulin (increasing its activity, thereby inhibiting beta-oxidation ) and by energy depletion via AMP-activated kinase (decreasing its activity, thereby increasing beta-oxidation).
Our body has distinct hormones for exercise and fasting. Adrenaline (also called epinephrine) is released by the adrenal gland and by peripheral neurons during exercise. It mostly acts on organs like the heart and muscle, but also on adipose tissue. Glucagon, by contrast, mainly acts on the liver and adipose tissue. Let us have a closer look how these hormones can activate Hormone sensitive lipase (Fig. 12).
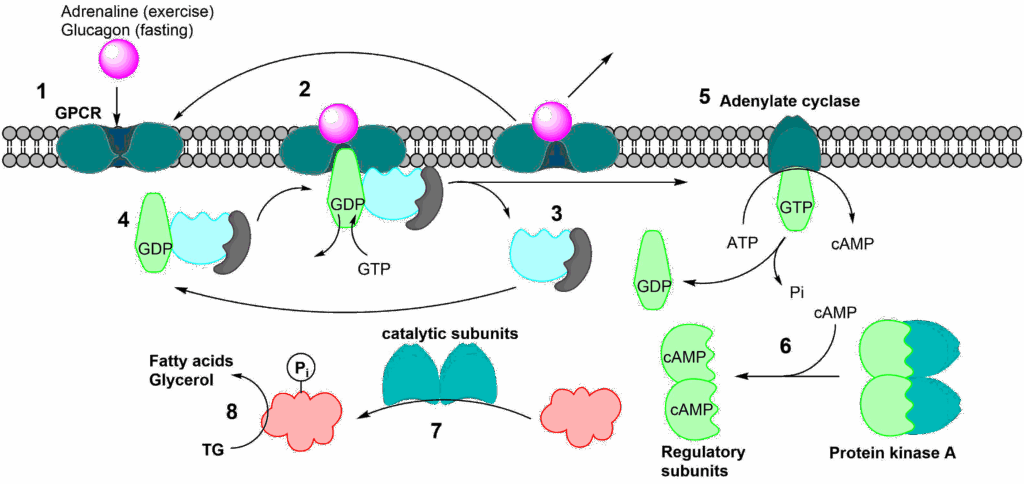
The process of initiating a physiological/metabolic program is called a signal transduction cascade. Fig. 12 shows the sequence of events. (1) The hormone (also called first messenger) binds to its receptor at the cell surface. This particular class of receptor is called a G-protein coupled receptor (GPCR). The term G-protein refers to a group of proteins that can bind GDP or GTP. In its inactive form GDP is found in the binding site. When GTP is in the binding site it gets hydrolysed to GDP. After binding of the first messenger, a conformational change occurs in the receptor, opening a binding site for a G-protein on the cytosolic side. When the G-protein binds to the receptor, GDP gets swapped for GTP (2). This causes the heterotrimeric G-protein complex to fall apart (3). The complex will reform once GTP in the alpha unit has been hydrolysed to GDP (4), thereby resetting the whole system. The alpha unit, which binds the GTP, is now activated and can interact with other proteins, most notably adenylate cyclase (5). Adenylate cyclase is activated by this process and catalyses the conversion of ATP to cyclic-AMP (cAMP) (Fig. 13).
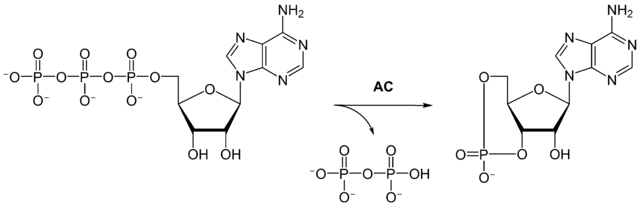
This small diffusible molecule is referred to as the second messenger and in turn activates other proteins, typically protein kinase A (6). The activation is caused by cAMP binding to the regulatory subunits, which then dissociate from the catalytic subunits. The liberated catalytic subunits can now phosphorylate the final target hormone-sensitive lipase (7), which hydrolyses fat to generate fatty acids and glycerol (8). In the detour you will learn that protein phosphorylation uses similar mechanisms as allosteric modulation. Please note the considerable amplification afforded by several steps in the cascade. Adenylate cyclase can produce many molecules of cAMP and protein kinase A can phosphorylate many enzyme molecules.

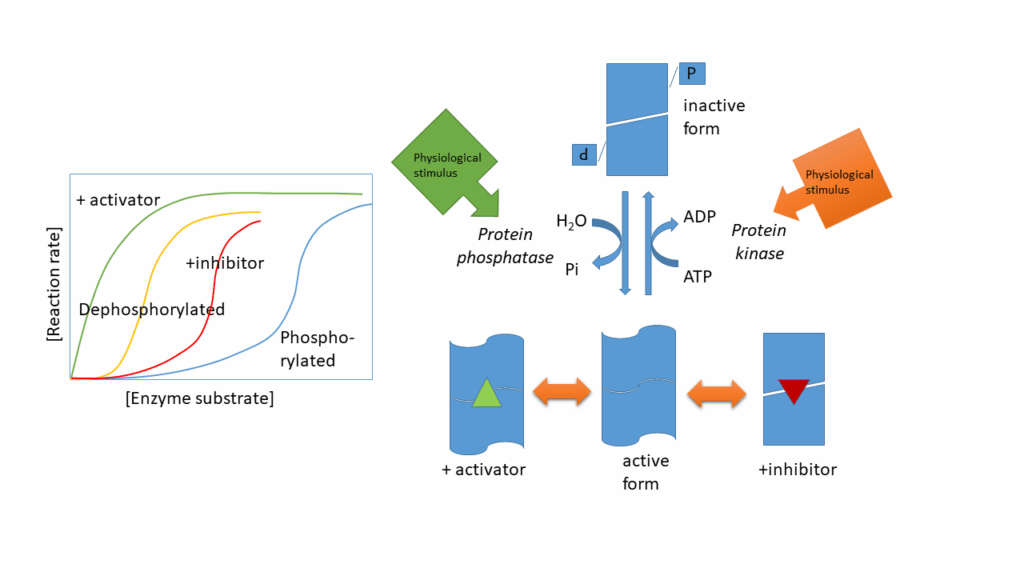
We discussed allosteric regulation of enzymes in chapter 7. Allosteric enzymes have an inactive (low affinity) and active conformation (high affinity), which are in equilibrium with each other. A positive allosteric effector stabilises the active conformation, while an allosteric inhibitor stabilises the inactive conformation. The same is often achieved by phosphorylation and dephosphorylation of serine, threonine and tyrosine residues in an enzyme (Fig. 14). Please note, there is no rule whether an enzyme is “switched on” by phosphorylation or “switched off’, it only changes its conformation. The change of conformation can be reversed by protein phosphatases. Both protein kinases and protein phosphatases can be regulated by signal transduction cascades.
As shown in Fig. 14 the active form of an enzyme that is regulated by protein phosphorylation is often additionally regulated by allosteric modulators.
The mobilisation of fat in adipose tissue is not only controlled by hormone sensitive lipase (HSL). Lipid droplets in adipose tissue are coated by large numbers of perilipin, an amphiphilic protein that binds to the surface of lipid droplets and prevents access by HSL. Protein kinase A also phosphorylates perilipin, thereby allowing access of HSL to the lipid droplet. A related lipase, adipose triglycerate lipase (ATGL), also gains access and helps with hydrolysis, because HSL does not hydrolyse all three ester bonds with equal efficiency. Mice lacking perilipin 1 have much higher triacylglycerol turnover and their fat mass is 70% reduced. Humans with certain rare inherited mutations in perilipin by contrast have elevated levels of lipid in blood and insulin-resistant diabetes. The insulin-resistant diabetes is probably caused by lipid deposits in other tissues, which are elevated because adipose tissue does not regulate fat synthesis and mobilization appropriately. High levels of fat in non-adipose tissue (called ectopic fat) causes lipotoxicity and insulin-resistant diabetes.
You want to burn some fat through exercise, in what metabolic state (fed, fasting) should you do this and why?
While fasting. When you exercise during fasting adrenaline and glucagon will work together to mobilise fat. After a meal insulin will promote the formation of fat and glucose is readily available to generate energy.
Arrange the following steps in fatty acid synthesis into the proper order.
1.Dehydration
2.Condensation
3. Release of C16 fatty acyl-CoA
4.Reduction of carbonyl-oxygen
5.Formation of Malonyl-ACP
- Formation of Malonyl-CoA
- Condensation
- Reduction of carbonyl-oxygen
- Dehydration
- Release of C16 fatty acyl-CoA
How is Acyl-Carrier Protein (ACP) similar to coenzyme A and where is it different?
ACP has an extension that is identical to part of coenzyme A. However, it earmarks acyl-groups for fatty acid biosynthesis. ACP is an integral part of fatty acid synthase and acts like a swinging arm. Coenzyme A is recognised by enzymes oxidising fatty acyl-CoA or metabolising acetyl-CoA.
What is the source of glycerol in Triacylglycerols (Fat)?
Glycerol can be derived from Glucose via the glycolysis intermediate dihydroxyacetone-phosphate or can be recycled from the breakdown of glycerolipids and fat.
What is the role of citrate in fatty acid biosynthesis?
Citrate is used as an indirect carrier for acetyl-CoA out of mitochondria, because acetyl-CoA is impermeable. Acetyl-CoA condenses with oxalocacetate to form citrate, which is shuttled to the cytosol. The reverse reaction (ATP citrate lyase) generates acetyl-CoA and oxaloacetate. Oxaloacetate can generate NADPH before returning as pyruvate back to the mitochondria. The presence of citrate in the cytosol also simulates acetyl-CoA carboxylase.
How do Adrenaline and Glucagon differ in their primary action sites within the body?
- Adrenaline primarily acts on heart and muscle while Glucagon acts on the liver.
- Adrenaline acts mostly on adipose tissue while Glucagon acts on muscles.
- Both Adrenaline and Glucagon primarily target the heart.
- Adrenaline mainly targets the liver, while Glucagon targets the heart.
Answer
Adrenaline acts mainly on the heart and muscles, but also affects adipose tissue, while Glucagon primarily targets the liver and adipose tissue. This distinction in action sites explains their roles in energy mobilization during different physiological states. The incorrect options suggest misplaced primary action sites for these hormones.
- Fat is an important fuel for many cells in the body.
- Fatty acid biosynthesis makes use of malonyl-CoA units, which are reduced by NADPH to form aliphatic fatty acids.
- Fat is mainly synthesized in the liver for delivery to other organs via VLDL particles. It is synthesized in adipose tissue for storage purposes.
- Lipoprotein lipase in endothelial cells releases fatty acids from VLDL.
- Fatty acid release from adipocytes is controlled by hormones. Perilipin and Hormone-sensitive lipase are key molecules controlling this release.
- Protein phosphorylation is a key mechanism to regulate the activity of enzymes
- Fig. 1 OpenStax College [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
- Fig. 2 By the author using ChemDraw
- Fig. 3 By the author using ChemDraw
- Fig. 4 By the author using ChemDraw
- Fig. 5 Wilhelm Trautschold (1815-1877), oil painting [Public domain], via Wikimedia Commons
- Fig. 6 By the author using ChemDraw
- Fig. 7 By the author using ChemDraw
- Fig. 8 By the author using ChemDraw
- Fig. 9 By the author using ChemDraw
- Fig. 10 Unnumbered photo by “Mike” Michael L. Baird [CC BY 2.0 https://creativecommons.org/licenses/by/2.0)]
- Fig. 11 By the author using Powerpoint
- Fig. 12 By the author using ChemDraw
- Fig. 13 Yikrazuul [Public domain], from Wikimedia Commons
- Fig. 14 By the author using Powerpoint