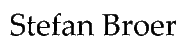Protein digestion and amino acid absorption
- Understand enzymatic catalysis using chymotrypsin as an example
- Recognise how rare disorders illustrate the role of amino acid transporters
- Understand the role of the liver and kidney in body metabolism
- Hydrolysis of proteins during digestion
- Mechanisms of enzyme catalysis
- Nutrient absorption of flow of nutrients inside the human body
- Role of rare disorders in the development of biological concepts
Thus far we looked at the digestion of carbohydrates and fats and learned that through oxidation and breakdown they were converted into acetyl-CoA, which was then fully oxidized by the TCA cycle and respiratory chain. Fat and carbohydrates contain only carbon, hydrogen and oxygen, all of which are also found in H2O and CO2. Amino acids contain nitrogen in addition, which as we will see in chapter 15, will be disposed of as urea. Another difference, between amino acid, carbohydrate and fat metabolism is the variety of amino acid structures. Different fatty acids and different carbohydrates have very similar structures. Fatty acids for instance only vary in length and the occurrence of double bonds. Carbohydrates are all built on the principle of 4-6 carbon atoms attached to a hydroxyl-group and a hydrogen. As a result carbohydrates and fatty acids can be metabolized by a single pathway, namely glycolysis and beta-oxidation, respectively. This will be different for amino acids. Before we look into the metabolism of amino acids we will first look into digestion and absorption.
Amino acids are categorized as nutritionally essential or non-essential. Humans can synthesize some amino acids, where only short biosynthetic pathways are required. We have lost biosynthetic pathways that need a significant input of ATP and instead acquire these amino acids through nutrition (Table).
|
Non-essential
|
Essential
|
Conditionally essential
|
|---|---|---|
|
Alanine
|
Histidine
|
Arginine (not in adults)
|
|
Asparagine
|
Isoleucine
|
Tyrosine (from Phenylalanine)
|
|
Aspartate
|
Leucine
|
Cysteine (S from methionine)
|
|
Glutamate
|
Methionine
|
|
|
Glutamine
|
Phenylalanine
|
|
|
Glycine
|
Threonine
|
|
|
Proline
|
Tryptophan
|
|
|
Serine
|
Threonine
|
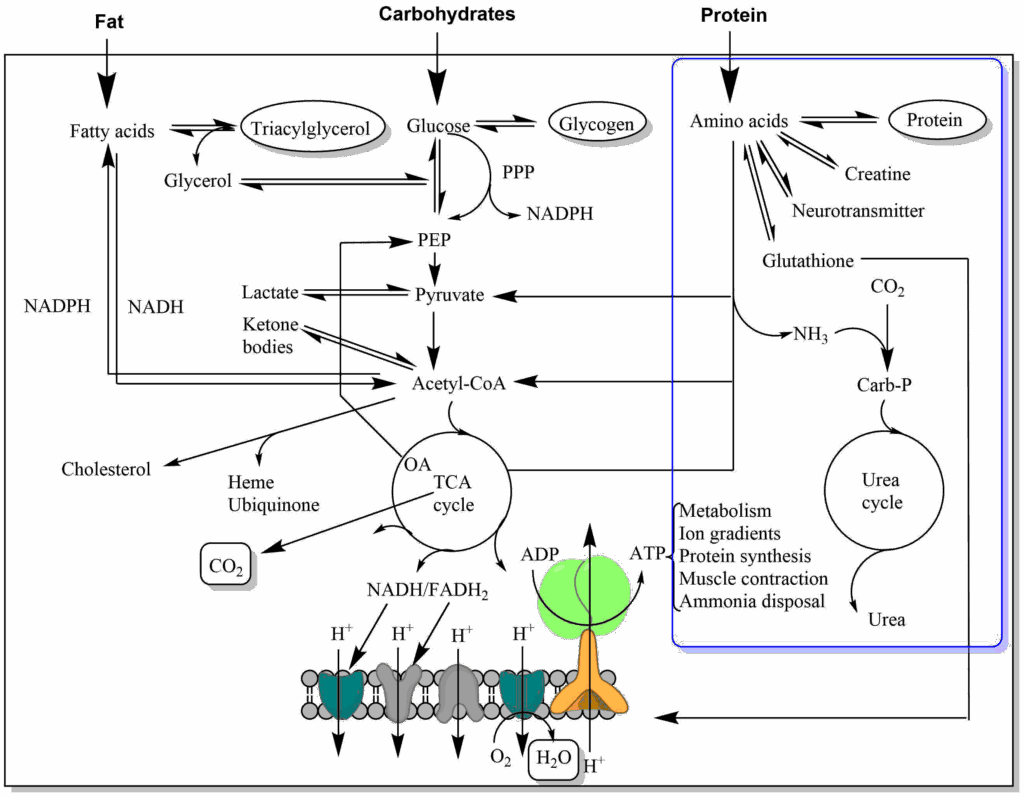
Proteins are polymers of amino acids. The peptide bond can be split by hydrolysis. This is achieved by a variety of proteases/peptidases (Fig. 1).
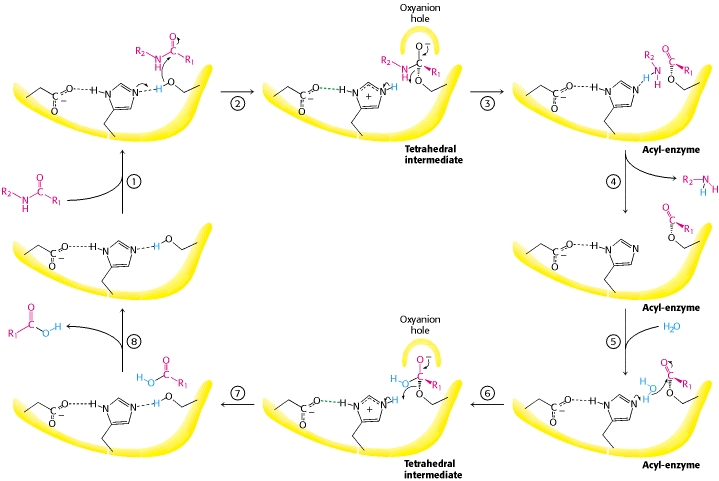
Protein digestion begins in the stomach. Pepsin is particularly well adapted to acidic pH and in addition protein is denatured by the acidic pH. The unfolded peptide-chain is easier to attack than a tightly fold protein. The acidic food slurry is neutralised by bicarbonate secretion of the pancreas. The pancreas also produces a variety peptidases that further split the protein fragments generated by pepsin into oligopeptides. A variety of peptidases are required, because of the different side chains of each amino acid. Each enzyme has a unique preference, such as before arginine, or before a large neutral amino acid etc. The remaining oligopeptides are further hydrolysed into individual amino acids, di- and tri-peptides by enzymes located in the brush-border of enterocytes. Carboxypeptidases cut from the carboxyterminus, while aminopeptidases cut from the amino-terminus. We will now have a closer look at one of the most well studied enzymes, the pancreas protease chymotrypsin (Fig. 2).
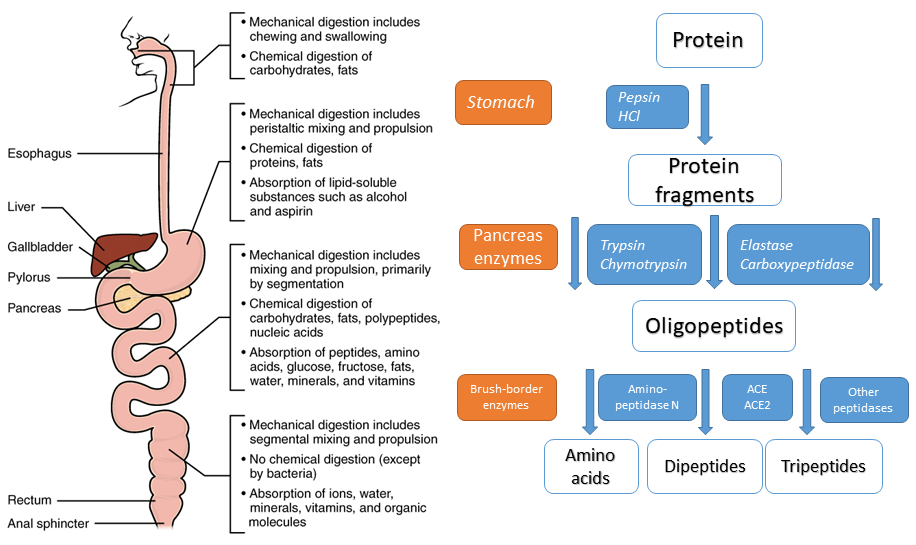
Chymotrypsin illustrates a number of features of protein design that we discussed in chapter 4. The primary structure shows a protein of 245 residues. Closer inspection, however, shows that the primary structure is interrupted at two places. (14-15 and 147-148 are missing). The reason for this design is the proteolytic activity of the enzyme. The pancreas stores digestive enzymes and would digest itself if they were continuously active. Instead, proteases are stored as pro-enzymes (also called zymogens, ending -ogen), which require partial proteolysis to become active. For instance, chymotrypsinogen is cleaved by trypsin to generate the active enzyme chymotrypsin. The partial proteolysis allows the precise alignment of the catalytical triade (Fig. 2 residues shown with side chain) that is the key of the catalytic mechanism (see video below). Thus, a particular folding pattern (tertiary structure) is required to bring these three residues together, which are at a distance in the primary structure. Chymotrypsin is largely made up of beta-sheets, which are shown as green arrows. Serine proteases are a large group of proteases characterized by serine in their active center.
Pro-enzymes and partial proteolysis are particularly important in the blood clotting cascade. Blood clotting is initiated by proteases, resulting in a cascade that activates other proteases. The fragmented protein fibrin is the final product, which aggregates into a mesh. Blood clotting is tightly regulated and should not occur without injury.
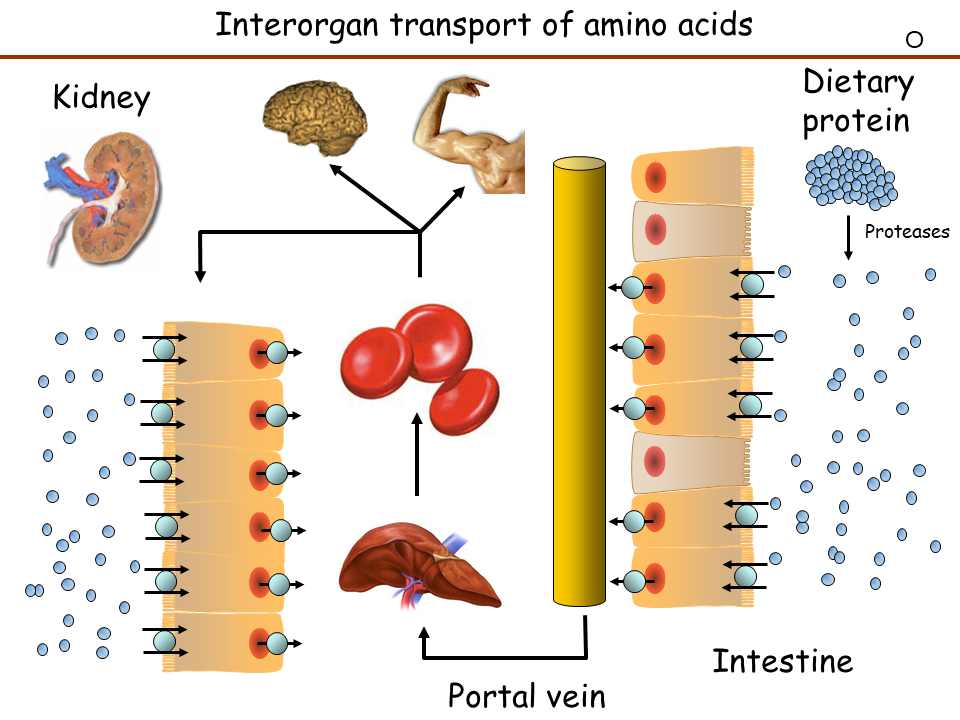
We now understand how proteins are digested into individual amino acids, di- and tripeptides. These are absorbed by the epithelial cells of the intestine through specific transporters (Fig. 4).
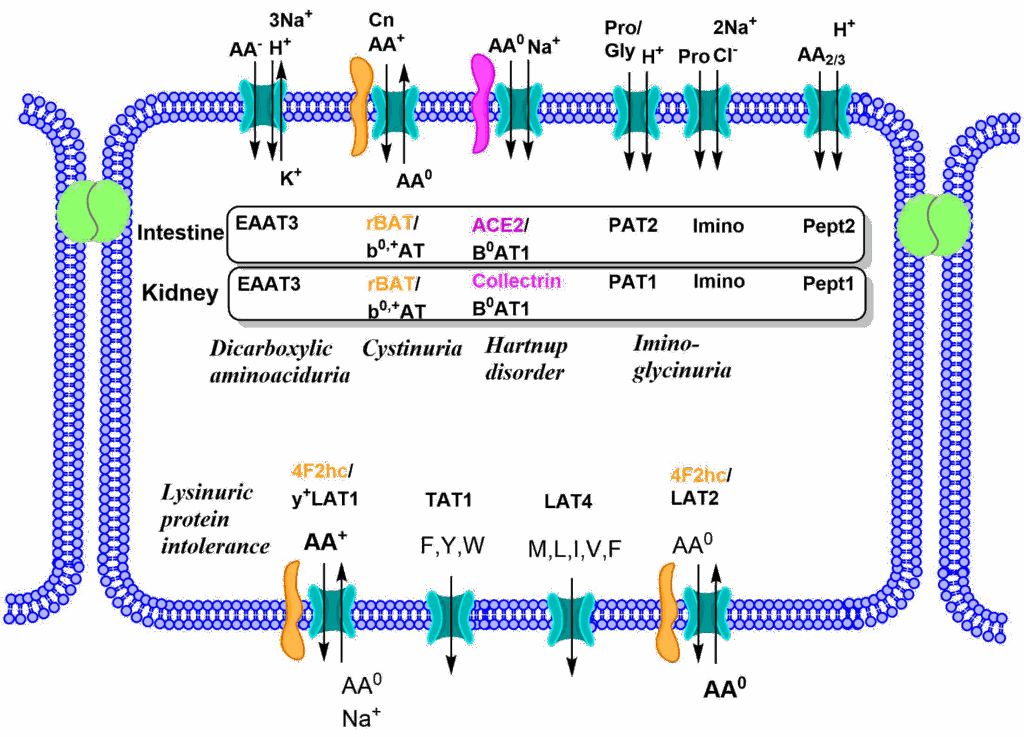
The absorption process is a bit more complicated than the one discussed for carbohydrates, because groups of similar amino acids are transported by a certain transporter instead of individual nutrients. As a result, transporters for neutral amino acids, cationic amino acids, anionic amino acids and glycine plus proline can be detected. Some transporters in addition are heteromeric complexes as indicated in Fig. 4. A combination of symport and antiport mechanisms are used to upload all amino acids across the apical membrane, while symporters and uniporters are used to release all amino acids across the basolateral membrane. Instrumental for the discovery of these transporters were a number of rare diseases listed in Fig. 4. These were mainly detected by a failure of these transporters in the kidney (see below). To understand this, we must have a brief look at blood flow (Fig. 5).
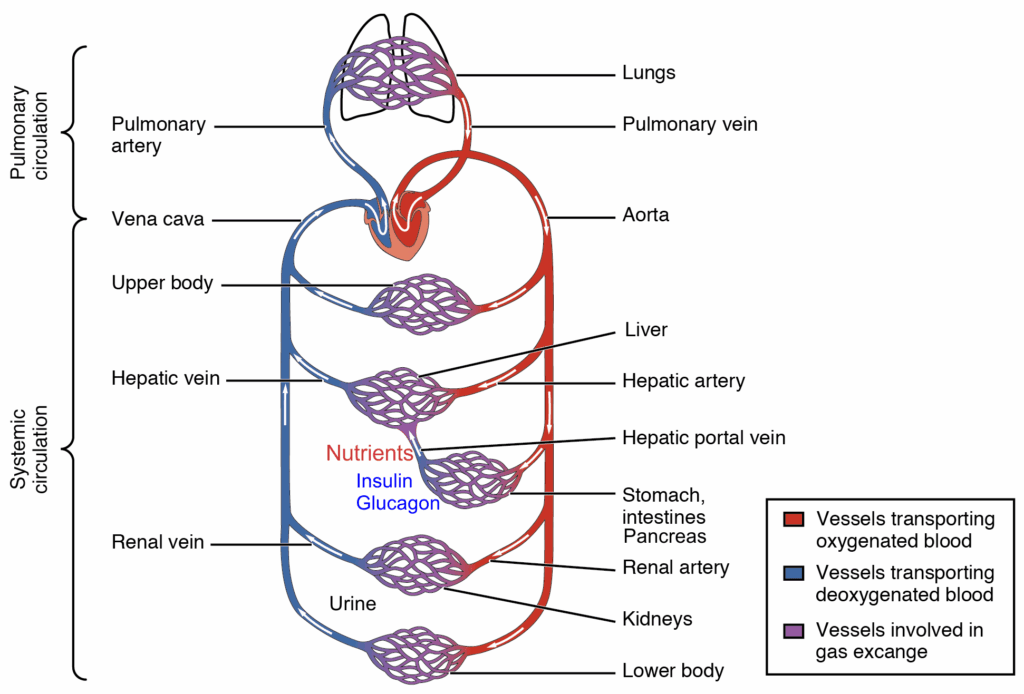
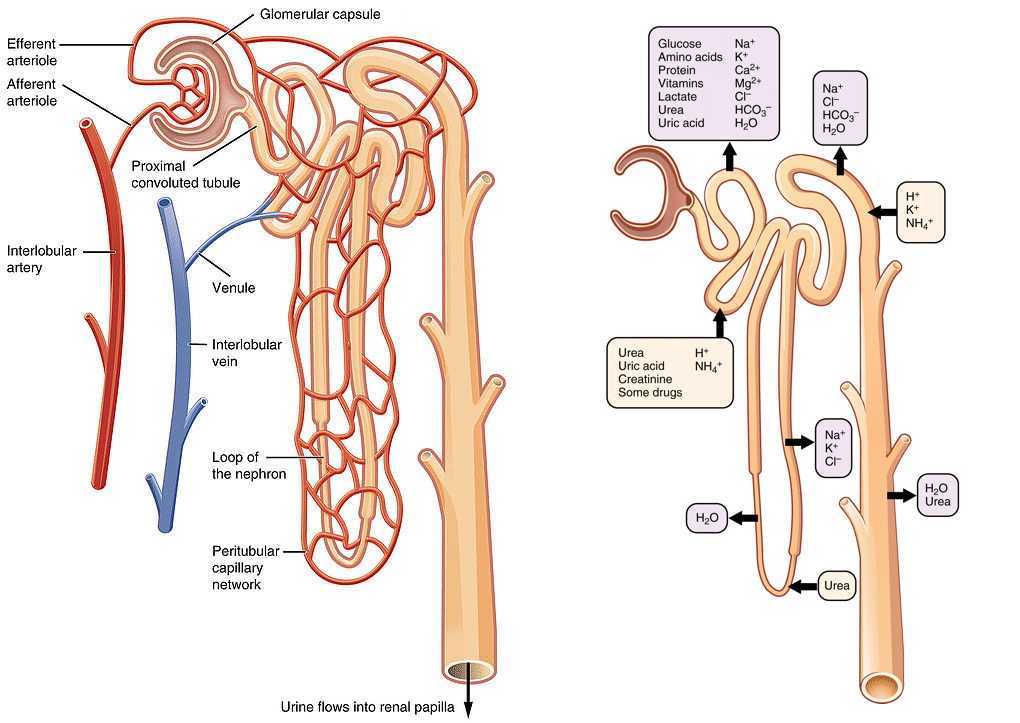
Nutrients are absorbed in the intestine and released into the portal vein, which leads to the liver. The portal vein also collects blood from the pancreas, where insulin and glucagon are secreted. The nutrients that pass through the liver will go through heart, pulmonary circulation, heart and be delivered to all organs through the systemic circulation including the kidneys. In the kidney blood is filtered in the glomerulus to generate primary urine (Fig. 6). The primary urine contains all low molecular weight components of the blood, while protein remains in the blood. To avoid wasting of water, nutrients, vitamins and salts, all important molecules and salts are reabsorbed in the tubules of the kidney. A total of 180l primary urine is generated every day of which 178l are reabsorbed including all valuable metabolites and salts (Fig. 6). Nutrients are absorbed in the early part of the tubulus called the proximal tubulus.
The reabsorption of amino acids is shown in Fig. 4. Specific transporters face the lumen of the tubule and capture all amino acids passing by. The rare disorders shown in Fig. 4 are caused by mutations in a specific transporter. This results in passing of the corresponding amino acids into the urine, called aminoaciduria.
At the age of 12 Eddie Hartnup was referred to the paediatric outpatient clinic at the Middlesex Hospital in early June, suffering from a severe sunburn-like red scaly rash on exposed areas of his skin. His mother said that she thought he was suffering from pellagra (niacin deficiency). His older sister, now aged 20, had been treated for pellagra some 10 years previously.
At the time he had a number of neurological signs that are not characteristic of pellagra. He had an unsteady gait, jerky arm movements and intention tremor. He also showed nystagmus and complained of double vision. His mother stated that several times during childhood he had suffered similar attacks, usually associated with the common winter-time illnesses such as flu, measles and mumps. He had always made a complete recovery after such attacks, which had not been associated with the pellagra-like rash.
A diet history showed that Eddie had a normal, and apparently adequate, intake of tryptophan and niacin. Therefore, dietary deficiency seemed improbable. Chromatography revealed excretion of a number of amino acids in his urine, with abnormally high concentrations of tryptophan, phenylalanine, tyrosine, leucine, isoleucine and valine. His urine also contained relatively high concentrations of a number of indolic compounds, including indoxyl sulphate (indican), indolylacetate, indolylacetamide and indolylacetylgIutamine, which are not detectable in the urine of normal subjects.
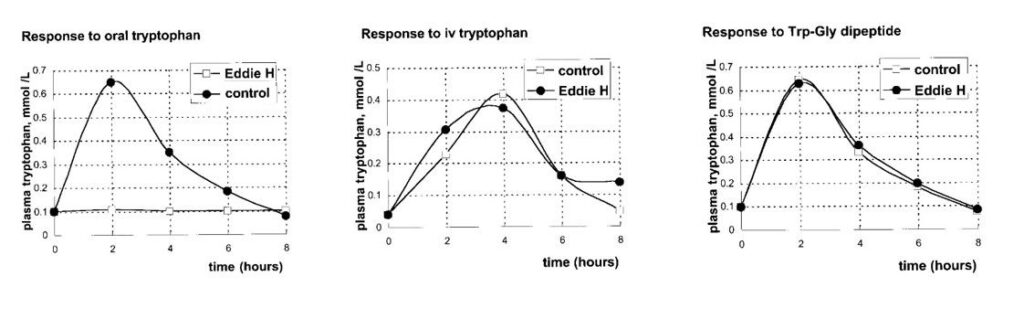
The figure below shows the effect on Eddie’s plasma tryptophan of giving him an oral or intravenous dose of tryptophan of 0. 5 mmol/kg body weight, or an equivalent amount tryptophanyl‑glycine by mouth.
What is the link between Niacin deficiency and Tryptophan? Although Niacin is a vitamin, NADH can be produced using tryptophan as a substrate. Our endogenous synthesis is insufficient and thought to contribute about half of the nicotinamide found in NADH and NADPH. As a result symptoms of Hartnup disorder are similar to those of Niacin deficiency.
A summary of nutrient flow through bodies is shown in Fig. 7. See Chapter summary for an explanation.
- Question
- Answer
Explain the response to oral tryptophan and tryptophan-peptide in the case of Eddie Hartnup (see case study).
Individual amino acids and peptides are absorbed by separate transporters. Eddie Hartnup has a defect in the transporter for neutral amino acids. His peptide transport is functional.
- Question
- Answer
Chymotrypsin is a serine protease. Serine is the key catalytic residue. Draw the mechanism of the chymotrypsin reaction and compare it with Fig. 3.
Serine mediates a nucleophilic attack on the carbonyl-group of the peptide bond. The resulting proton is stored on the neighbouring histidine. A covalent intermediate is formed to the enzyme, while the peptide bond is broken. The released C-terminal peptide picks up the proton. Water hydrolyses the covalent intermediate re-protonates histidine. The proton is returned to serine-OH.
- Question
- Answer
What is the role of the kidney tubule in maintaining nutrient, water and ion levels?
The kidney tubule is the place where individual components of the primary urine (formed in the glomerulus) are recaptured. In the proximal tubule all nutrients are reabsorbed and returned to the blood. Water and ions are absorbed all along the tubule.
- Question
- Answer
Why are proteases stored as proenzymes?
Proteases are non-specific and can destroy any protein. If digestive enzymes would be active during storage they would damage the pancreas. Limited proteolysis activates zymogens only after their release from the pancreas.
- Question
- Answer
Where are the major proteases involved in protein digestion located?
Protein digestion begins in the stomach (pepsin), the main digestion occurs in the small intestine mediated by pancreas peptidases such as chymotrypsin, trypsin. Brush-border peptidases break down final peptides into individual amino acids, di- and tri-peptides.
- Dietary protein is digested by a variety of proteases into amino acids, di- and tri-peptides.
- Group-specific transporters absorb amino acids, di- and tri-peptides into epithelial cells and pass them through basolateral transporters into the blood stream.
- Nutrients are collected by the portal vein and brought to the liver.
- Amino acids in the circulation are filtered in the kidney. Valuable nutrients are reabsorbed in the proximal tubulus and passed back into the blood stream.
- Mutations of amino acid transporters can be recognised by overflow of amino acids into the urine.
- Fig. 1 By the author using Powerpoint using OpenStax College image [CC BY 3.0]
- Fig. 2 By the author using ChemDraw and PyMol
- Fig. 3 JM Berg, JL Tymockzo, L Stryer [Copyrighted free use], via Wikimedia Commons
- Fig. 4 By the author using ChemDraw
- Fig. 5 OpenStax College [Public domain], via Wikimedia Commons
- By the author using Powerpoint