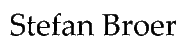17 Regulation of glucose metabolism
- Understanding the feeding fasting cycle
- Understand how hormones control metabolic programs
- Appreciate individual variations of metabolic control
- Hormonal regulation of metabolism
- Metabolic state during the feeding/fasting cycle
- Glycaemic control
- Glucose as a reactive aldehyde
- Fuel use during exercise
We have looked at many metabolic pathways that control breakdown and synthesis of metabolites. In this chapter we want to integrate these pathways into a bigger picture of metabolic control and appreciate what can go wrong. Fig. 1 shows the course of plasma glucose and the changes of two major hormones (insulin and glucagon) after a meal. Please note that neither insulin nor glucagon ever goes all the way to 0. Both hormones are constantly released to some extent. It is rather the insulin/glucagon ratio that controls fuel metabolism.
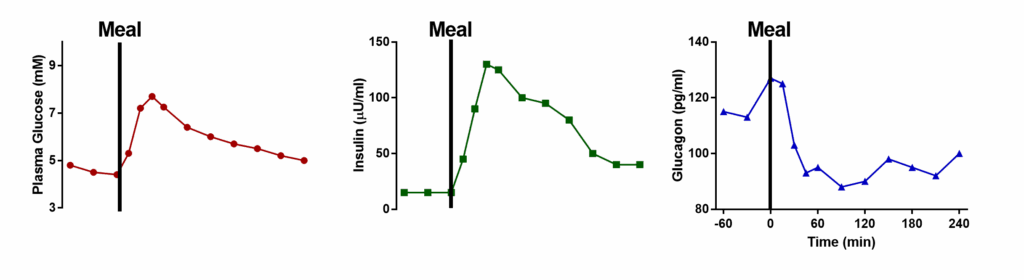
In general, insulin controls nutrient deposition after a meal (the fed state, Fig. 2), while glucagon mobilizes fuel reserves in the post absorptive state (fasting state, Fig. 4). Easy to remember is that insulin polymerizes nutrients (glucose -> glycogen, amino acids -> protein, acetyl-CoA -> fat) and glucagon does the opposite.
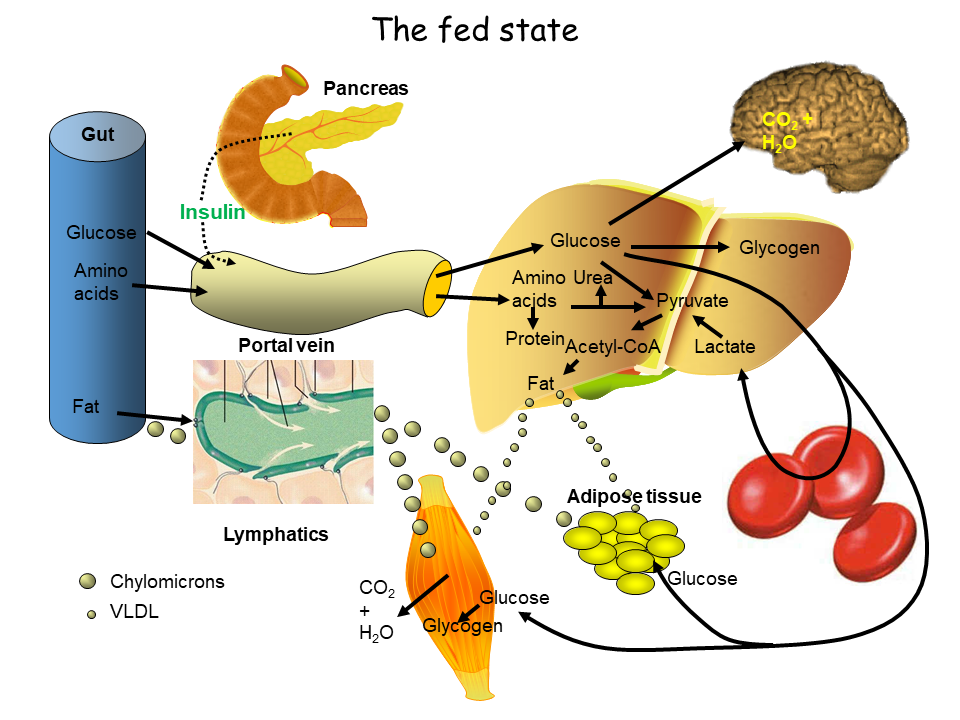
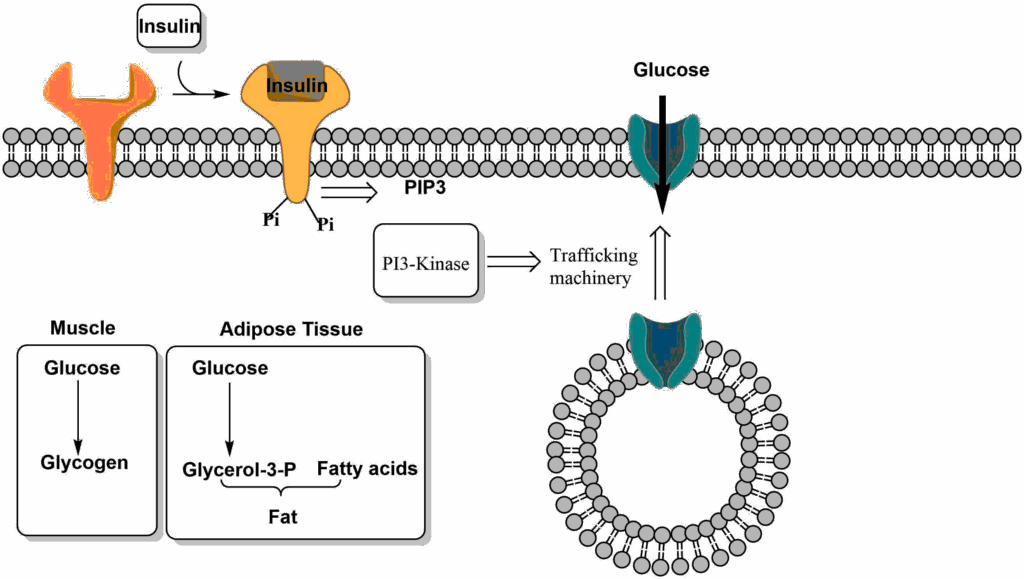
In the fed state all three macronutrients are absorbed by the intestine and reach the liver through the portal vein. Depleted glycogen stores are refilled. Excess glucose passes through the liver and is used by other tissues. In particular, glucose is taken by muscle to refill glycogen stores and by adipose tissue for the generation of glycerol-3-phosphate, the backbone for fat biosynthesis. In the liver excess glucose is converted into fat and delivered to other organs as VLDL particles. The efficient removal of glucose by these mechanisms underlies the drop of blood glucose back to fasting levels within 2-3 h after a meal with muscle being the major sink. The other macronutrients are also stored. The intestine packages fat into lipoprotein particles called chylomicrons, which are transferred to adipose tissue and muscle for storage. Amino acids are used to synthesize protein; excess amino acids are oxidized to generate energy. This metabolic program is governed by insulin. Insulin also initiates the use of glucose by muscle and adipose tissue after a meal by bringing their glucose transporters to the cell surface (Fig. 3). Between meals, glucose transporters are stored in vesicles below the plasma membrane. Binding of insulin to its receptor initiates the insulin pathway (chapter 8), which activates PI3-kinase (Phosphatidylinositol-3-kinase). Via a variety of proteins, the trafficking machinery is activated resulting in fusion of the vesicles with the plasma membrane (Fig. 3). Mice lacking this insulin-responsive glucose transporter are highly insulin resistant, because they cannot efficiently remove glucose from blood.
Once all nutrients are deposited and metabolized after a meal, which typically takes 2-4h, blood glucose levels return to normal. Fig. 4 summarizes the use of fuel after a 24h fast. During the day we replenish nutrients every 4h or so but between dinner and breakfast our body has to mobilize reserves to maintain blood glucose. The use and provision of fuel after 24h of fasting is shown in Fig. 4. Adipose tissue and muscle are the providers of fuel. Muscle amino acids are used for gluconeogenesis and Adipose fat is used to provide fatty acids and glycerol. Fatty acids can be used as fuels particularly by the heart and muscles. In the liver fatty acids are converted into ketone bodies, which can be used by any organ. Glycerol is converted into glucose. Glucose is the main fuel for the brain and blood cells and is derived from gluconeogenesis and glycogen.
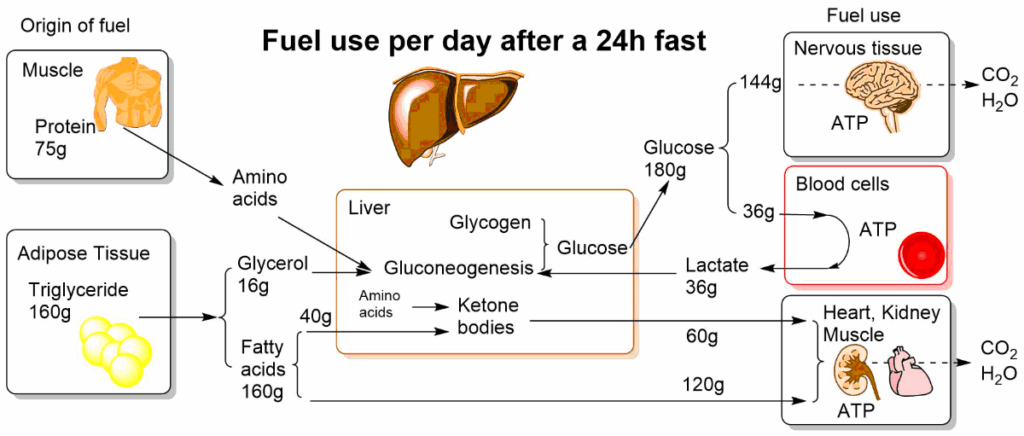
In the fasting state glucagon is the governing hormone. It causes breakdown of glycogen in liver to maintain blood glucose concentration above 4 mM. In adipose tissue it initiates lipolysis, which provides fatty acids for muscle cells and glycerol as a source of gluconeogenesis. The other main sources for gluconeogenesis are amino acids derived from muscle and lactate.
The picture is oversimplified, because there are quite a few more hormones that fine-tune the feeding/fasting cycle, such as cortisol, growth hormone and peptides that act on the brain to stimulate or reduce appetite (leptin, ghrelin, etc.). Another important hormone is adrenaline, which prepares our body for energy expenditure (exercise, fight or flight response). Like glucagon it mobilises fuel reserves, but mostly for the benefit of exercise, thus heart and muscle are its main target organs. Let us explore the actions of the three key metabolic hormones on the pathways we have discussed so far.
|
Hormone
|
Pathway
|
Liver
|
Muscle
|
Adipose tissue
|
|---|---|---|---|---|
|
Insulin
|
Glucose uptake
|
None
|
+
|
+
|
|
Insulin
|
Glycolysis
|
+
|
+
|
+
|
|
Insulin
|
Gluconeogenesis
|
-
|
n.a.
|
n.a.
|
|
Insulin
|
Glycogen
|
+
|
+
|
n.a.
|
|
Insulin
|
Fat
|
VLDL(+)
|
+
|
++
|
|
Glucagon
|
Glucose uptake
|
None
|
n.a.
|
None
|
|
Glucagon
|
Glycolysis
|
-
|
n.a.
|
-
|
|
Glucagon
|
Gluconeogenesis
|
+
|
n.a.
|
n.a.
|
|
Glucagon
|
Glycogen
|
-
|
n.a.
|
n.a.
|
|
Glucagon
|
Fat
|
-
|
n.a.
|
-
|
|
Adrenaline
|
Glucose uptake
|
None
|
+
|
None
|
|
Adrenaline
|
Glycolysis
|
None
|
+
|
None
|
|
Adrenaline
|
Gluconeogenesis
|
+
|
n.a.
|
n.a.
|
|
Adrenaline
|
Glycogen
|
-
|
-
|
n.a.
|
|
Adrenaline
|
Fat
|
n.a.
|
-
|
-
|
We have discussed the regulation of each individual pathway in the corresponding chapters. Together they are well tuned to provide us with energy during the day and all of the night. But what happens when we consume too much food over extended period of times and become obese? This is an area of intensive research, and the precise mechanisms are still very controversial. What is not controversial is that over-consumption numbs our cells to the action of insulin. Normally cells are sensitive to insulin and as we have seen above, insulin mobilises glucose transporters to bring them to the cell surface. The transporters then remove glucose from the bloodstream, and it can be stored as glycogen. In obese people cells become
insulin resistant. It is thought that elements of the insulin signal transduction cascade are less responsive to the signals that propagate from the insulin receptor (see video).
There are several consequences. Glucose transporter mobilisation to the membrane is reduced. The switching from gluconeogensis to glycolysis in the liver is incomplete. The switch from glycogenolysis to glycogenesis is incomplete. Overall this causes the blood glucose spike after a meal to be much higher (>10 mM, compare with Fig. 1), the return to fasting levels to be much longer (>3 h) and fasting blood glucose to be higher (> 6 mM). Long-lasting high blood glucose is thought to be underlying much of the long-term side effects of diabetes. Glucose is an aldehyde in its linear form. Normally very little of the linear form is present in blood, but in diabetes this is elevated. Glucose as an aldehyde can react with protein (Fig. 5). This results in a modification called advanced glycation endproducts or AGE‘s.
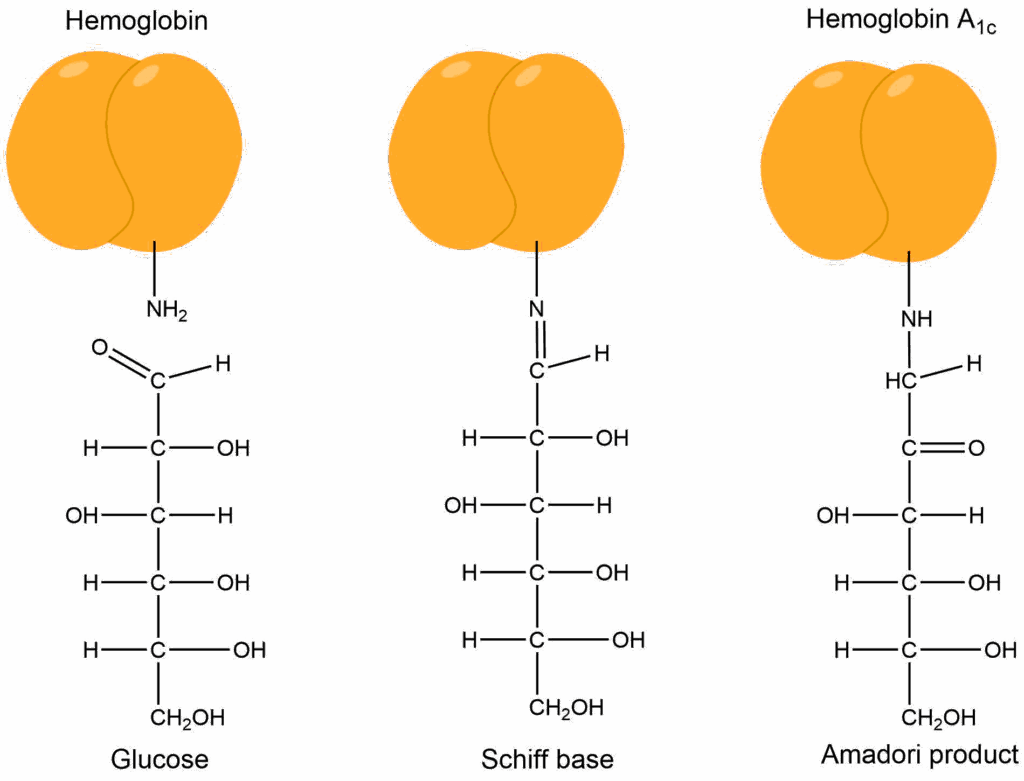
The reaction of glucose with hemoglobin (referred to as glycated hemoglobin or HbA1c) is used diagnostically to determine the long-term average blood glucose concentration. Glucose can react with any protein, particularly in blood vessels. This causes vascular damage, which then causes heart attacks, ulceration of limbs, chronic kidney disease and cataracts. Thus, glucose spikes and elevated glucose levels are considered damaging in the long-term. However, the response of blood glucose levels to food intake varies between food items (see Fig. 1 chapter 8), but also between individuals. Watch the following video about glycemic responses in humans and their individual variability. This could lead to new ways of managing type 2 diabetes.
One particular interesting case of nutrient is fructose. Fructose is metabolised to fructose-1-phosphate, which is split by aldolase B into dihydroxyacetone phosphate and glyceraldehyde (Fig. 6). This has profound metabolic consequences.
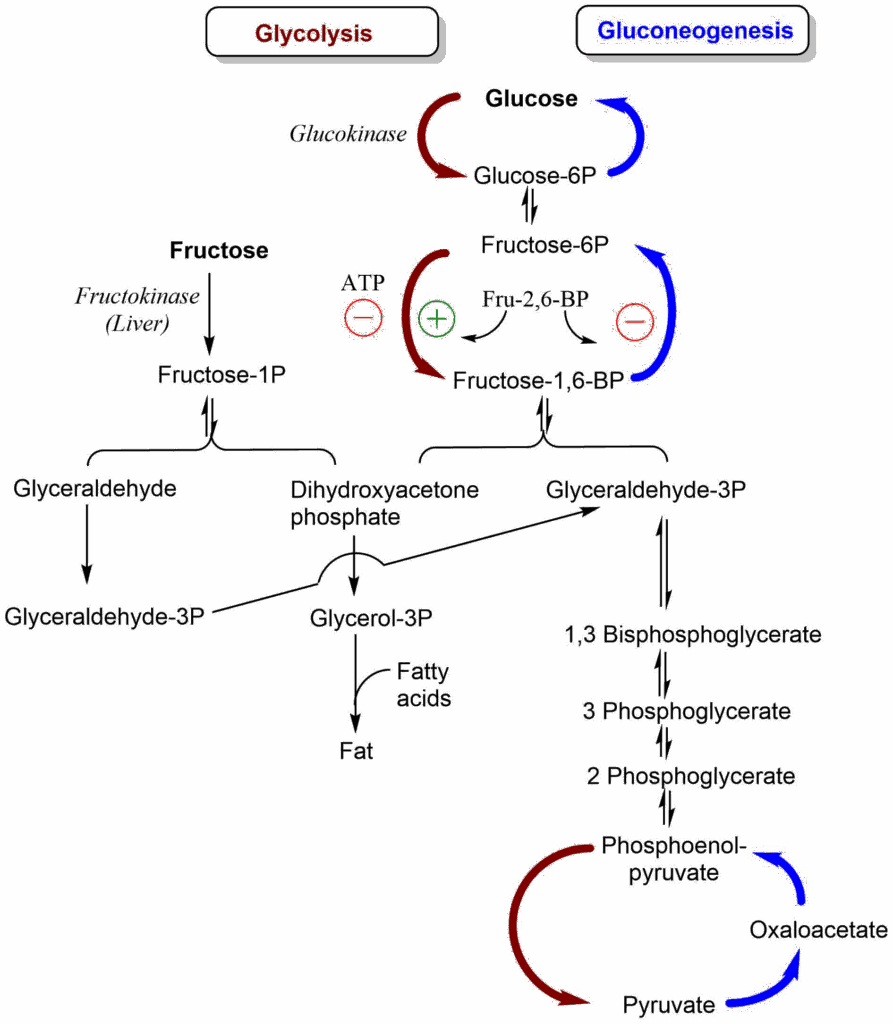
Fructose metabolism is less regulated by insulin or energy state, because it bypasses the tightly regulated phosphofructokinase reaction. Fructose is mainly metabolized in liver because of the expression of fructokinase, which is only found in this organ. It generates glyceraldehyde and dihydroxyacetonephosphate. The former is converted into glyceraldehyde-3-phosphate merging with glycolysis, but the latter is converted into glycerol-3-phosphate, the backbone for fat production. Recent research suggests that a significant part of the fat production from fructose involves acetate produced by the intestinal microflora from fructose.
Fat accumulation in liver is highly correlated with insulin resistance in liver. There are several theories how fat accumulation causes insulin resistance (lipotoxicity), which we discussed earlier (see Stop & Think in chapter 12 and Real-World application in chapter 10). Due to the production of glyceraldehyde-3P and dihydroxyacetone phosphate, fructose can also increase the glucose output by the liver in the fed state, particularly when insulin resistance is rising.
While our body nowadays has to fight with nutrient excess, evolution has selected for very strong responses to nutrient limitation (Fig. 6). Adrenaline, growth hormone and cortisol are released when blood glucose levels fall too low but are also released in the morning to prepare us for the day. We have already looked at metabolic responses to adrenaline above.
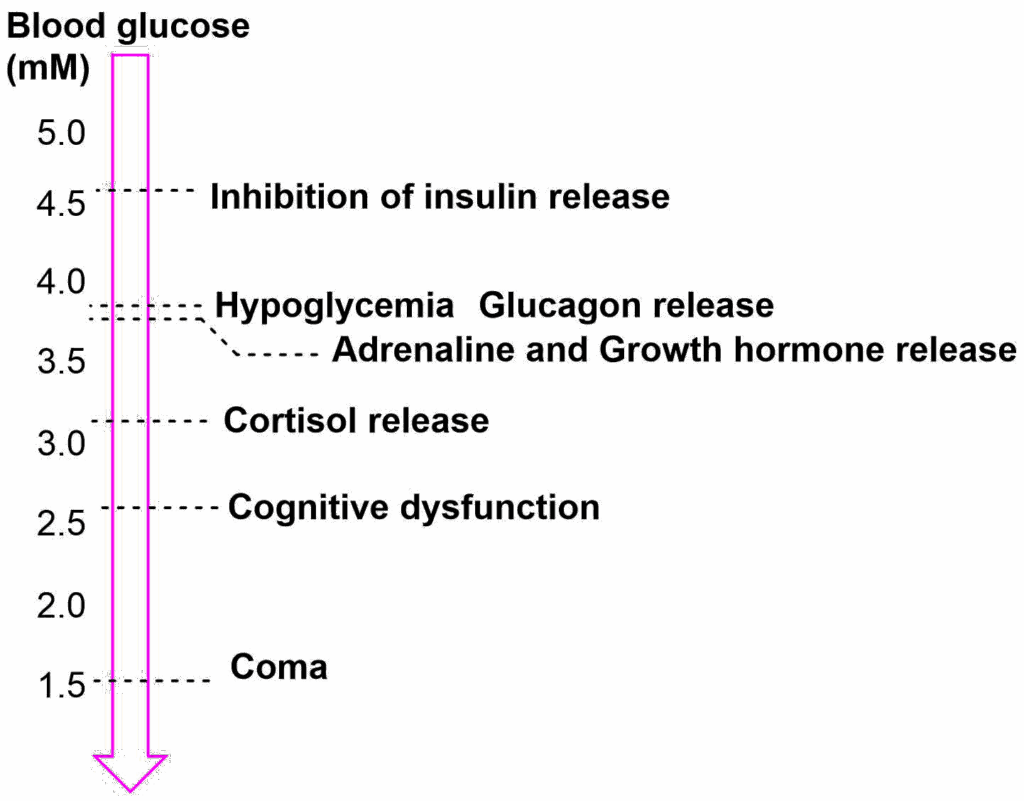
Cortisol is a stress hormone that changes a number of metabolic processes. To prepare us for the day it is released at dawn before we wake up and increases gluconeogenesis and insulin resistance (to extend the time of elevated glucose).
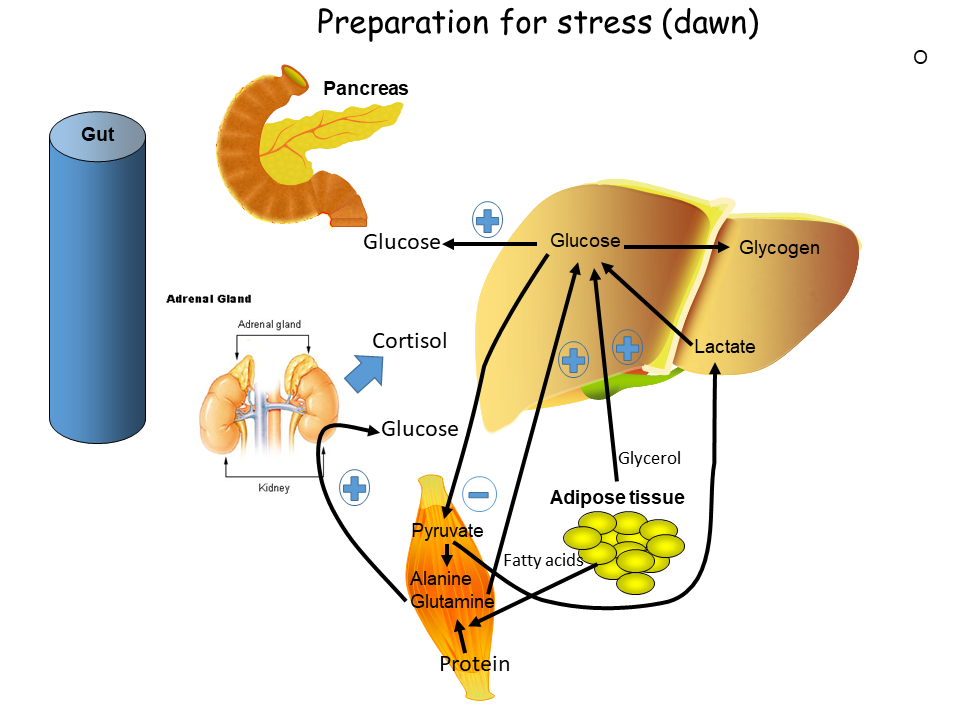
However, when cortisol is released, glucagon has already activated gluconeogenesis through allosteric mechanisms and protein phosphorylation. But we can increase gluconeogenesis even further by increasing the production of enzymes involved in gluconeogenesis. Glucocorticoids bind to the glucocorticoid receptor, which is a DNA-binding protein. The receptor dimerizes and activates gene promoters that contain a glucocorticoid response element (GRE), by recruiting and activating the transcriptional machinery (Fig. 8).
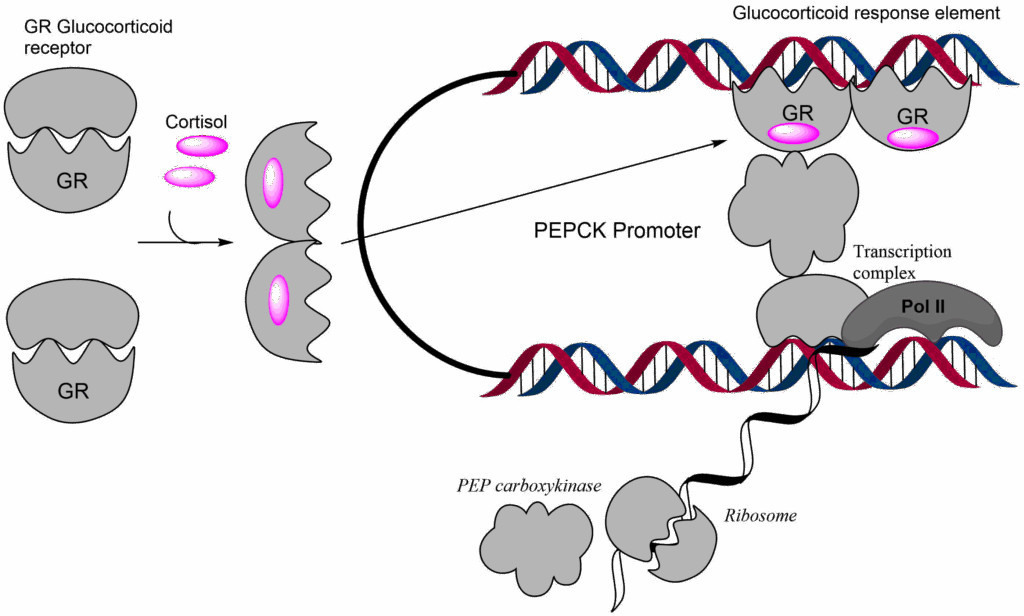
The actions of cortisol in the morning explain why people with diabetes wake up with elevated levels of blood glucose. Even in a person with type 2 diabetes it could be expected that glucose is used up overnight, and its concentration falls to fasting levels, but this is not the case. In fact, type 2 diabetes is typically diagnosed by fasting glucose levels >6 mM. Growth hormone as its name suggests is important to regulate growth in children but is also a metabolic hormone in adults. It stimulates lipolysis and thus reduces the reliance on glucose as a fuel.
Lastly, we want to explore how our metabolism works during exercise. Adrenaline is the main hormone released during exercise and its metabolic actions are listed in the Table further up in this chapter. Essentially adrenaline causes glycogenolysis (in muscle) and lipolysis through the production of cAMP. It also increases the flux of glucose into muscles although by a different pathway than insulin, the release of which is blunted by adrenaline. The main fuels for all tissues are glucose, lactate, palmitic acid (fatty acids), glutamine, 3-hydroxybutyrate (ketone body) and branched-chain amino acids. In the table below the fuel preferences for different organs (in mice) are listed under fasting and exercise conditions.
|
Tissue
|
Fasting
|
Exercise
|
|---|---|---|
|
Quadriceps
|
PA>Lac>3OHBut
|
Lac>PA>3OHBut>Glc
|
|
Soleus
|
PA>3OHBut>Lac
|
Glc>>PA=Lac>3OHBut
|
|
Heart
|
PA>>Lac>3OHBut
|
PA>>Lac>3OHBut
|
|
Kidney
|
PA=Gln=Lac>3OHBut
|
3OHBut>PA=Lac=Gln
|
|
Liver
|
Lactate>PA>Gln
|
Lactate>PA>Gln
|
Perhaps surprising is the dominance of fatty acids as a fuel in mice during fasting; in muscle glucose becomes the predominant fuel during exercise. This is mainly caused by the breakdown of glycogen. Muscle does not only produce lactate but also uses lactate when sufficient oxygen is available. The Heart has a strong preference for fatty acids, hardly using glucose at all. The kidney uses glutamine as a major fuel but can also use it to generate glucose. Fuel usage by the liver and heart is not affected by exercise.
- Question
- Answer
Insulin regulates metabolism. Indicate the response (+, 0, -) of the listed pathways to insulin
- Glucose uptake in muscle
- Gluconeogenesis
- Glycolysis in liver
- Glycogen synthesis
- Fatty acid biosynthesis
- Glucose uptake in muscle +
- Gluconeogenesis –
- Glycolysis in liver +
- Glycogen synthesis +
- Fatty acid biosynthesis +
- Question
- Answer
Glucagon regulates metabolism. Indicate the response (+, 0, -) of the listed metabolic pathways to glucagon.
- Glycogenolysis in liver
- Glycogenolysis in muscle
- Glucose uptake in muscle
- Gluconeogenesis
- Glycolysis in liver
- Fatty acid biosynthesis
- Lipolysis
- Glycogenolysis in liver +
- Glycogenolysis in muscle 0
- Glucose uptake in muscle 0
- Gluconeogenesis +
- Glycolysis in liver –
- Fatty acid biosynthesis –
- Lipolysis +
- Question
- Answer
Adrenaline regulates metabolism. Indicate the response (+, 0, -) of the listed pathways to adrenaline.
- Glycolysis in muscle
- Glycogenolysis in liver
- Glycogenolysis in muscle
- Glucose uptake in muscle
- Gluconeogenesis
- Glycolysis in liver
- Fatty acid biosynthesis
- Lipolysis
- Glycolysis in muscle +
- Glycogenolysis in liver 0
- Glycogenolysis in muscle +
- Glucose uptake in muscle +
- Gluconeogenesis –
- Glycolysis in liver +
- Fatty acid biosynthesis –
- Lipolysis +
Question
Hemoglobin 1Ac is used as a diagnostic marker in people with diabetes, why?
Answer
Elevated concentration of blood glucose increases the opportunity for the aldehyde-form of glucose to react with proteins. Hemoglobin 1Ac is used as a diagnostic marker, but glycation will occur in many proteins and cause long-term damage. Keeping Hb1Ac low reduces long-term complications of diabetes.
Question
Why is fructose considered to be nutritionally worse than glucose?
Answer
Fructose is metabolised by the liver. Its metabolism merges with glycolysis at the triose-phosphate stage. This bypasses the most important regulatory step of glycolysis in liver. Excess fructose is thus readily converted into fat, causing fatty liver disease in the long term.
- Fig. 1 Drawn after: Unger RH: Glucagon physiology and pathophysiology. N Engl J Med285 : 443–449,1971
- Fig. 2 By the author using Powerpoint
- Fig. 3 By the author using ChemDraw
- Fig. 4 By the author using ChemDraw
- Fig. 5 By the author using ChemDraw
- Fig. 6 By the author using ChemDraw
- Fig. 7 By the author using Powerpoint
- Fig. 8 By the author using ChemDraw