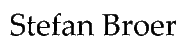16 Pentose-Phosphate-Pathway and oxygen metabolism
- Understand the consequences of oxidative metabolism
- Understand the mechanisms to defuse oxygen radicals
- Appreciate the role of reactive oxygen species in ageing
- Generation of oxygen radicals by the respiratory chain
- Defence against oxygen radicals
- Mitochondrial theory of ageing
- Oxygen radicals and heart disease
- Radicals as second messengers
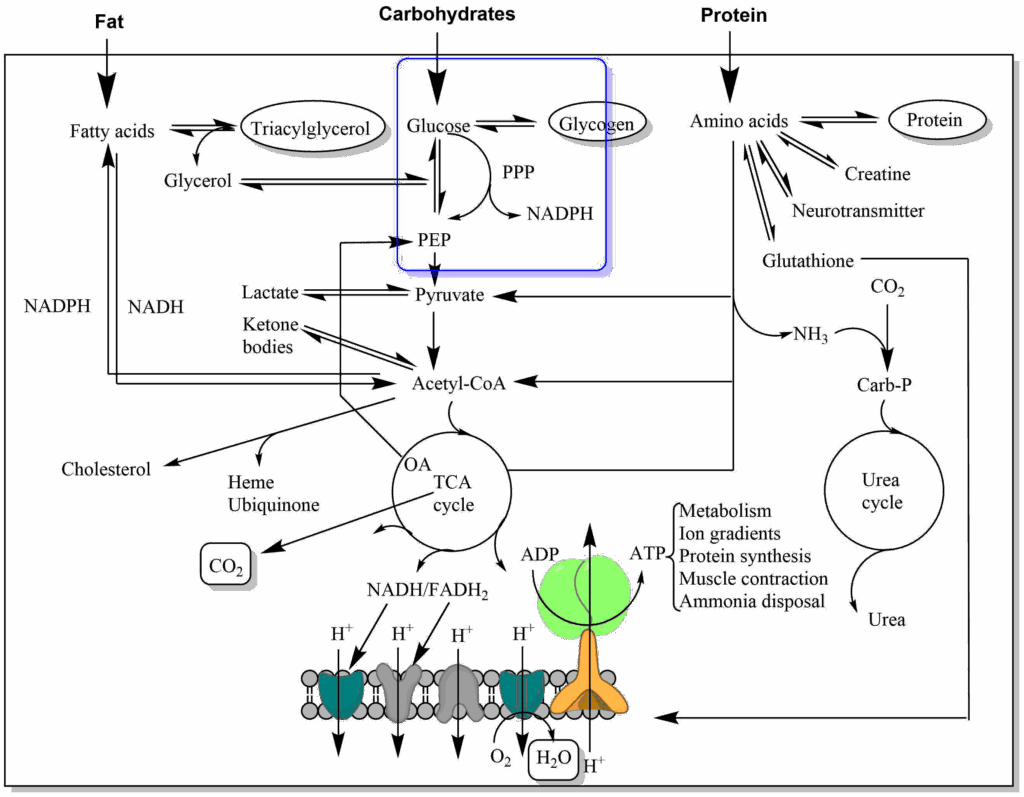
In previous chapters we have discovered different metabolic pathways that glucose can use (Fig. 1). In this chapter we will discover another pathway of glucose metabolism that generates NADPH (for biosynthetic processes) and Ribose-6-phosphate (for nucleotide biosynthesis).
You have previously discovered that NADPH is essential to provide reducing equivalents to anabolic reactions such as fatty acid biosynthesis. However, this is not the only role that NADPH serves, it is essential to defend us against oxygen radicals. What are oxygen radicals and why do we need to fight them?
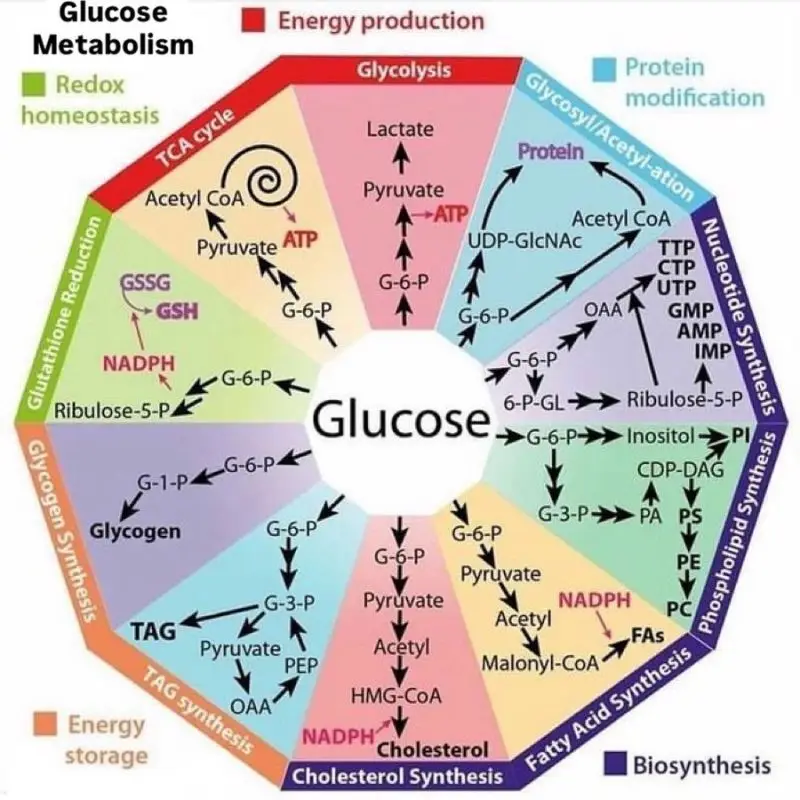
The respiratory chain (electron transport chain) moves electrons between different redox centers (chapter 10). At the same time, oxygen is always present as the final electron acceptor. What prevents oxygen from picking up electrons before the formation of water at complex IV? Most of the time the electrons are shielded in metal complexes, such as iron-sulfur clusters and cytochromes that are buried within a protein. However, electrons are also transferred onto ubiquinone (coenzyme Q), which floats in the lipid bilayer, and this can react with oxygen at the semi-quinone radical stage (Fig. 2).
We have used Fig. 2 before to show all reactions that provide electrons to the ubiquinone pool (chapter 10). Unavoidably, any of these reactions can also generate oxygen radicals by transferring an electron to oxygen forming superoxide anions (O2–). However, all complexes of the respiratory chain can produce oxygen radicals, when they transfer or receive electrons from other elements of the respiratory chain.
Radicals are chemically reactive, because they have an unpaired electron and as much as possible want to attract another electron, which results in the formation of hydrogen peroxide, a very reactive molecule in its own right. Transfer of a further electron generates the hydroxyl radical (see Fig. 5 below). Collectively oxygen radicals are referred to as reactive oxygen species (ROS).
Why are oxygen radicals so damaging?
Because radicals are very reactive, their lifetime is short, and they react with components in their vicinity. Lipid peroxidation (Fig. 3) at double bonds is particularly frequent. This causes membrane leakage, which in turn causes swelling of cells and cell compartments such as ER, lysosomes and mitochondria. In mitochondria lipid peroxidation can cause opening of the mitochondrial permeability transition pore (MPTP), which is normally only activated during apoptosis and thus resulting in cell death.
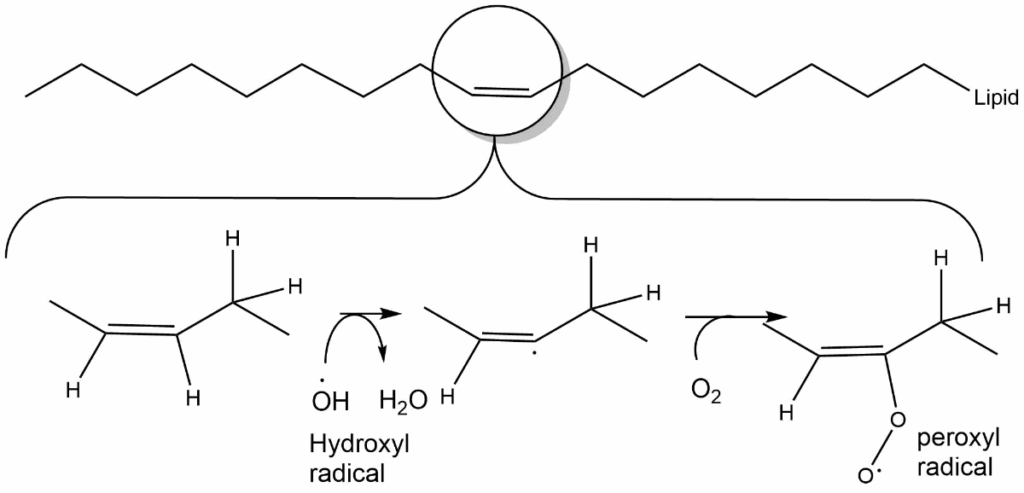
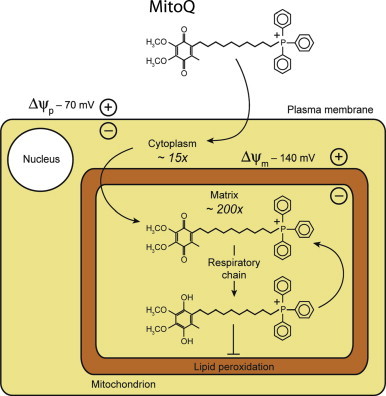
Ischemia is defined as reduced blood flow to an organ. This occurs after an infarct or during storage of organs for transplantation. Paradoxically, reinstating the blood flow, for instance by thrombolysis or grafting of the transplant, causes further damage to the organ. This is caused by a burst of oxygen radicals upon reperfusion, which damages the mitochondria and can result in cell death. Clinical trials are performed to see whether addition of free radical scavenging molecules can reduce the damage. To target the molecules into the mitochondria a hydrophobic cation is used that accumulates inside mitochondria because of the highly negative membrane potential.
It is no surprise, therefore, that all organisms have developed very sophisticated mechanisms to defuse oxygen radicals. They are found both in mitochondria and the cytosol. The main enzymes are superoxide dismutase, catalase and glutathione peroxidase (Fig. 4). Please note that superoxide dismutase does not fully detoxify oxygen radicals because it generates hydrogen peroxide. It is only in combination with catalase or glutathione peroxidase that water is generated. Glutathione is a tripeptide Glu-Cys-Gly. The cysteine residue can quench radicals by forming a disulfide bridge after catching two electrons. In order to restore glutathione it is reduced by NADPH.
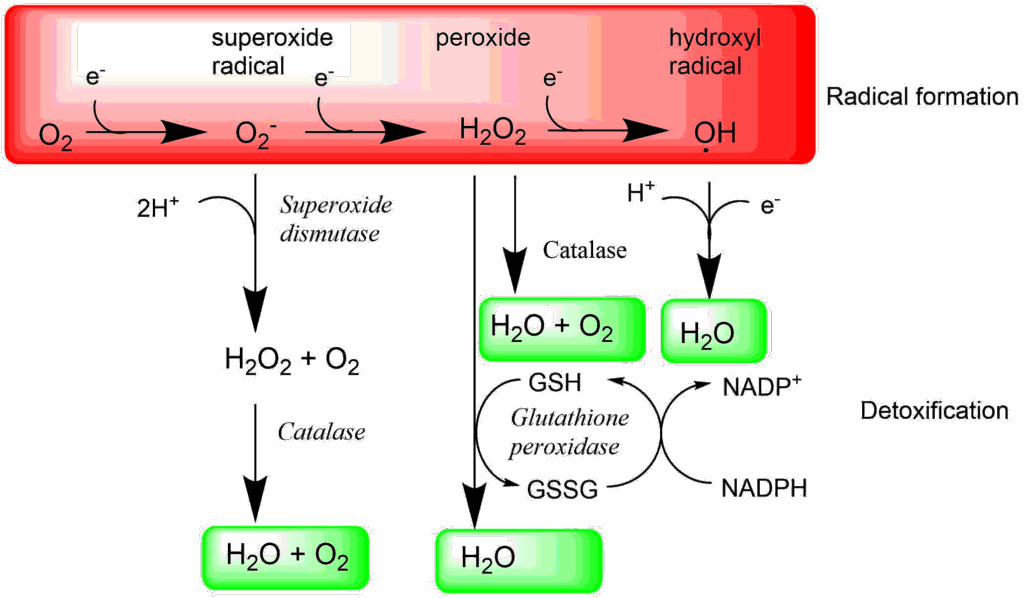
The detoxification pathways outlined in Fig. 4 are all occurring in the cytosol. Glutathione peroxidase 4 (GPx4) is an enzyme that specifically detoxifies lipid peroxides (L-OOH) to lipid-hydroxides (L-OH). Inhibition or inactivation of GPx4 results in a specific form of cell death called Ferroptosis, illustrating its role in detoxification.
The major producer of NADPH is the Pentose-Phosphate Pathway (PPP), which branches away from glycolysis after the generation of glucose-6-phosphate (Fig. 6).
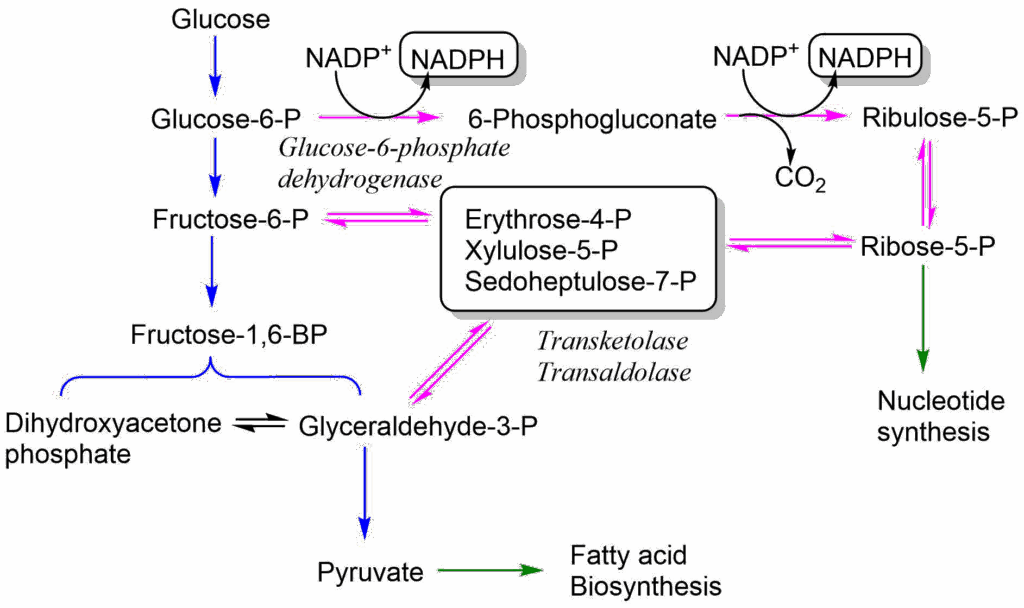
In the oxidative arm of the PPP glucose-6-phosphate is oxidised in two enzymatic steps to generate two molecules of NADPH. In the second step CO2 is generated, as well, resulting in the pentose Ribulose-5-phosphate, which is converted to Ribose-5-phosphate. The pathway is thus also the generator of all ribose subunits for de novo DNA and RNA synthesis. The pathway has several modes and Ribose-5-phosphate can alternatively be converted back into intermediates of glycolysis, namely fructose-6-phosphate and glyceraldehyde-3-phosphate. The pathway is not shown here in any more detail, because it is quite complicated (i.e. 3 x C5 ribose generates 2 x C6 fructose and 1 x C3 glyceraldehyde). The overall equation is:
3 Glucose-6-P + 6 NADP+ + 3H2O → 2 Fructose-6P + 1 Glyceraldehyde-3P + 3CO2 + 6NADPH+
Radicals are not only damaging cells, but they are also used as signaling molecules and for the destruction of invading bacteria by neutrophils. An important signaling mechanism involves the generation of nitric oxide from the precursor arginine in endothelial cells lining blood vessels (Fig. 7). Nitric oxide can diffuse into neighbouring smooth muscle cells where it activates guanylate cyclase. Guanylate cyclase generates cGMP from GTP. Like cAMP, cGMP is a second messenger that activates a protein kinase, called protein kinase G. This causes blood vessel relaxation and reduces blood pressure. Nitroglycerin spray is commonly used to relax blood vessels going to the heart. In the aqueous environment of the blood stream nitroglycerin generates nitric oxide. It thus replaces the function of the endothelial cells and causes relaxation of constricted heart vessels.
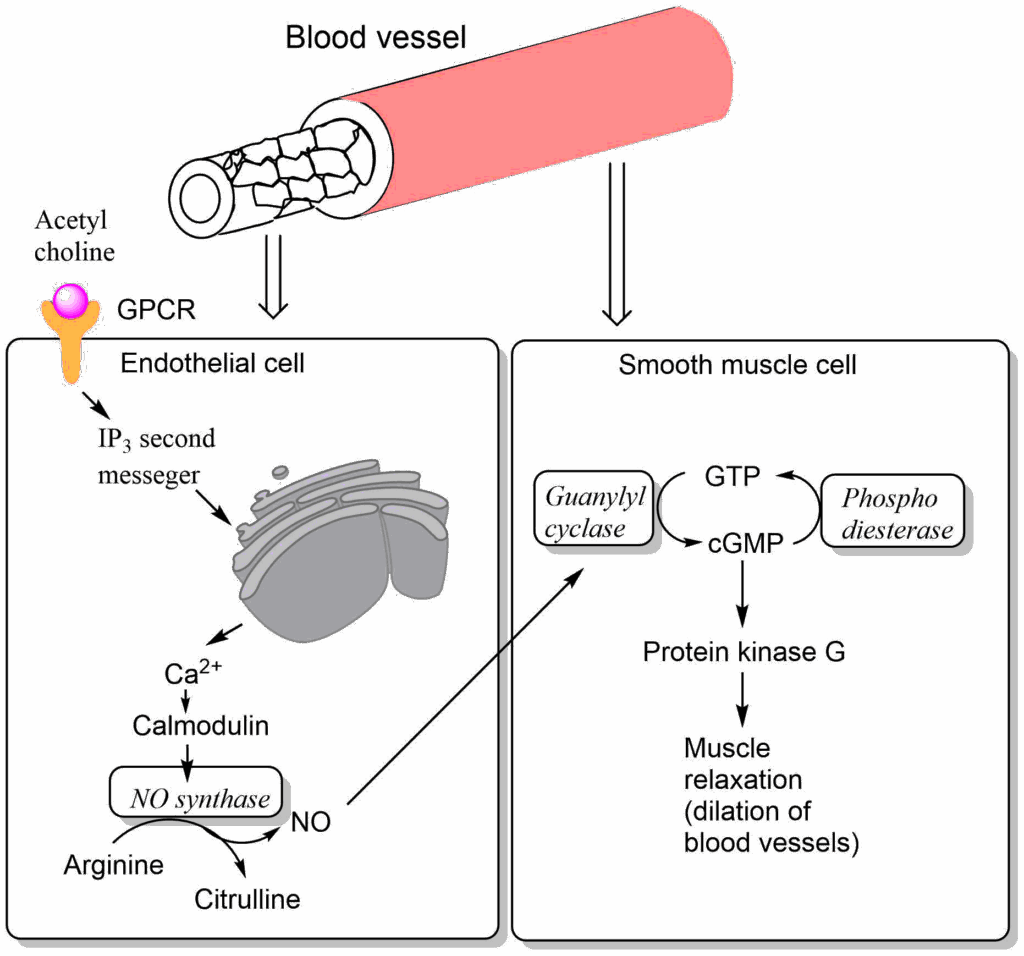
Viagra is an inhibitor of phosphodiesterase, which is required for the breakdown of cGMP. It thus extends the lifetime of cyclic GMP. It is a specific inhibitor of an isoform found in the penis where blood vessel relaxation causes an enhanced blood flow into the penis causing an erection.
Another complex side of radicals is there possible role in the ageing process. Because of the continuous generation of radicals it appears likely that not 100% of them are always detoxified. Thus over a long time damage can accumulate (tear and wear theory of ageing). We have seen in chapter 12 that oxidised cholesterol in LDL and VLDL particles can accumulate in the walls of blood vessels, thereby slowly generating atherosclerosis. Atherosclerosis is a sign of advanced age. More principal is the question what causes ageing in all cells in our body? In the Table below processes are listed that increase with age and are thought to associated with ageing:
Table: Hallmarks of ageing
|
Process
|
Causes
|
Consequences
|
|---|---|---|
|
DNA damage and mutations
|
Radiation, UV light, repair mistakes
|
Cancer, precancerous cell clones
|
|
Telomere shortening
|
Cell division
|
Cellular senescence
|
|
Accumulation of misfolded proteins
|
Reduced autophagy, proteins prone to misfolding, protein adducts |
Amyloids (Alzheimers, Parkinsons), AGE's
|
|
Epigenetic alterations
|
Increased DNA methylation
|
Reduced gene activity
|
|
Accumulation of senescent cells
|
Telomere shortening, mitochondrial dysfunction |
Heart disease, Kidney disease, inflammation |
|
Mitochondrial dysfunction
|
ROS, reduced autophagy
|
Heart disease, neurodegeneration
|
|
Resistance to signalling
|
Signal overload
|
Diabetes
|
Is there a clock ticking in every cell counting the time until we die? The mitochondrial theory of ageing proposes that oxygen radicals damage the mitochondrial DNA and the nuclear DNA (Fig. 8). For instance, the nucleobase guanine can be converted to 8-hydroxyguanine by hydroxyl radicals, which isomerises to 8-oxo-7,8-dihydroguanine. This modified nucleobase causes errors in base pairing, because it can pair with adenine. DNA proofreading and repair in mitochondria has much less fidelity than in the nucleus, thus over time mutations accumulate. Importantly, mitochondria have an error-prone DNA polymerase, thus mutations occur even without oxidative damage. Assembly of mutated and non-mutated proteins in the respiratory chain causes reduced efficiency of ATP generation and perhaps even more generation of reactive oxygen species. Lipid peroxidation can cause opening of the mitochondrial permeability transition pore, which initiates apoptosis. Thus, over time mitochondria degenerate and are lost. In the end the whole cell deteriorates.
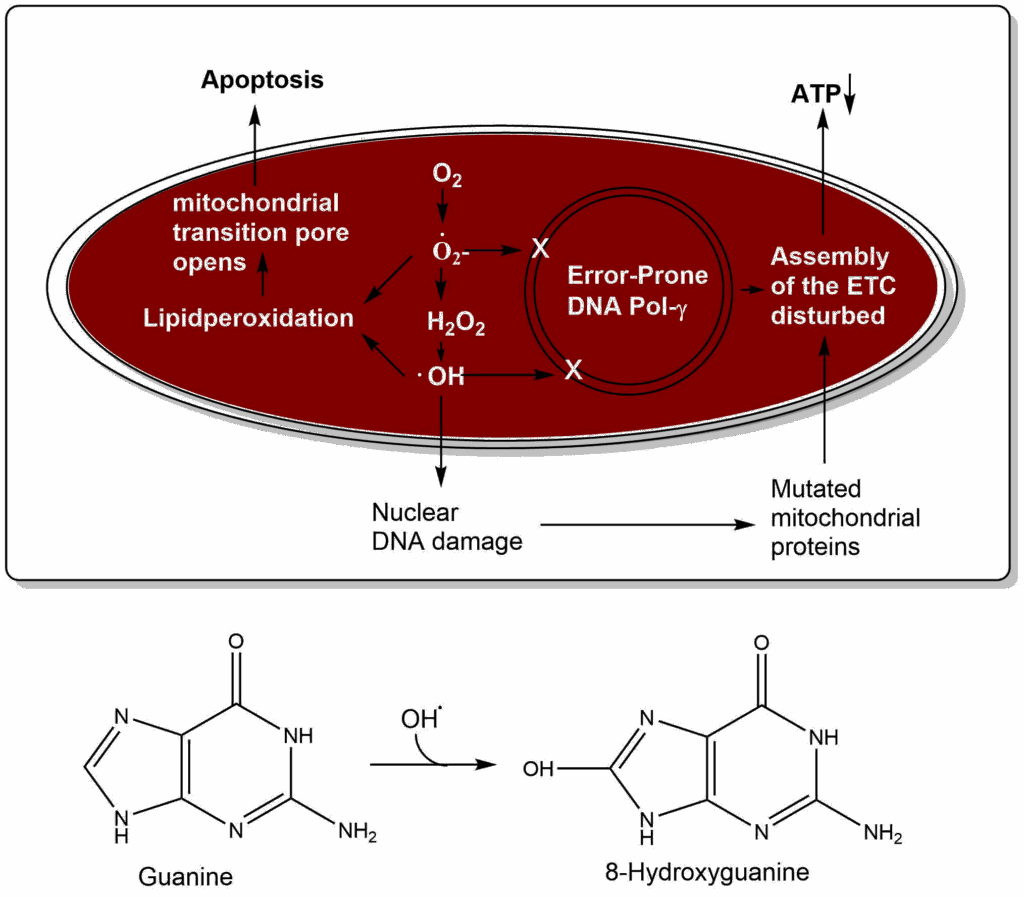
The mitochondrial theory of ageing is attractive, because it explains a number of observations. First, mitochondria are still encoding genes. It has been argued that evolution had sufficient time to completely move all mitochondrial DNA into the nucleus. However, if mitochondrial DNA serves as a timekeeper, it has an important evolutionary function. Second, mice expressing a mutated mitochondrial DNA polymerase with an even higher mutation rate show premature ageing. Third, the use of antioxidants as supplements to extend lifespan has shown little effect in higher organisms.
The limited success of antioxidants to prevent signs of ageing may also result from the presence of endogenous antioxidants. We have already discovered the enzymes that defuse oxygen radicals. Our cells have even more arsenal available to combat radicals, namely ascorbic acid (Vitamin C, water soluble) and Tocopherol (Vitamin E, lipid soluble) (Fig. 9).
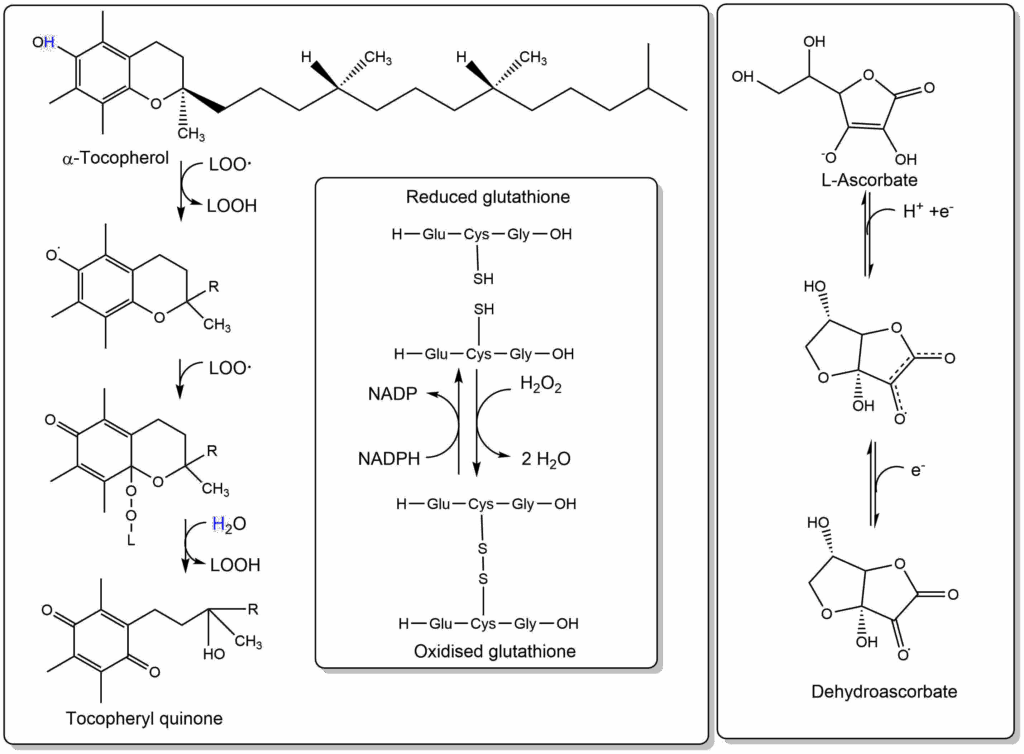
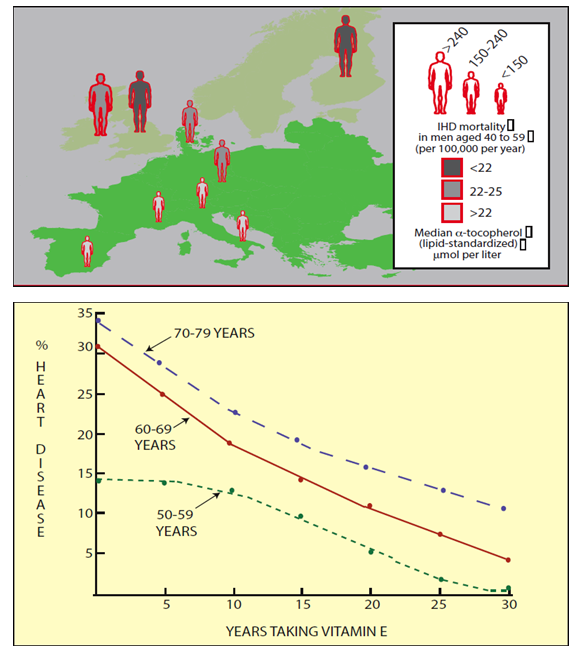
Vitamin E in particular is embedded in membranes and can quench radicals associated with lipids. It reacts with two lipid-peroxyradicals and converts them into the corresponding peroxides. Thus, it can also help to prevent oxidation of lipoprotein particles. There is indeed strong epidemiological evidence that elevated blood levels of Vitamin E reduce the incidents of heart disease (IHD in Fig. 10 upper panel). Taking Vitamin E as a long-term supplement also reduces the frequency of heart disease (Fig. 10 lower panel).
Ascorbic acid is better known as Vitamin C. Vitamin C deficiency results in scurvy where connective tissue starts to become fragile resulting in ulceration of the gums, and hemorrhages caused by fragile blood vessels and other symptoms. The effects are due to inability of the enzyme prolyl-4-hydroxylase to modify proline residues in collagen into hydroxyproline, which is required for its proper folding. The enzyme carries out a side reaction, decarboxylating 2-oxoglutarate to succinate during which its iron atom in the catalytic center becomes oxidised. The enzyme can only be regenerated by vitamin C reducing the iron atom. Eating fruits prevents scurvy due to their content of vitamin C.
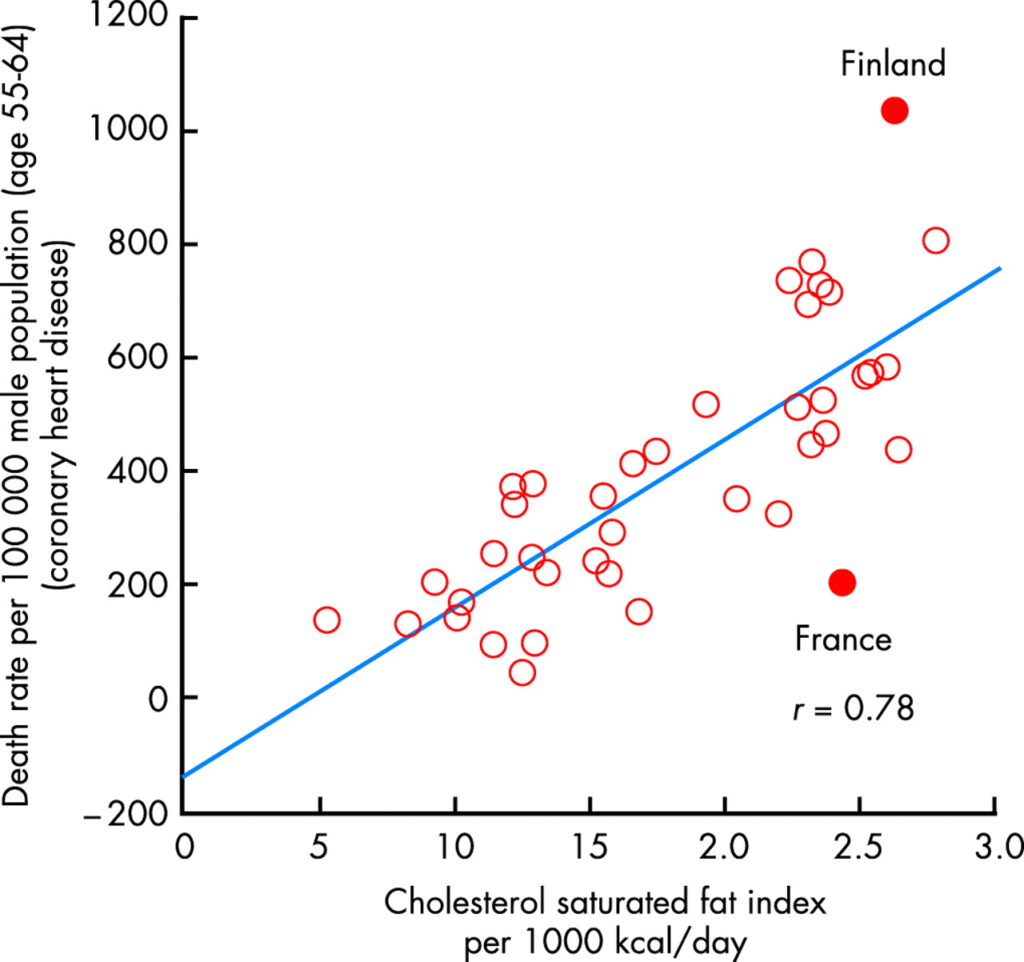
Our nutrition contains additional antioxidants, particularly in plant derived food. Red wine for instance has a complex mixture of polyphenols derived from grape skins. These function as antioxidants. The so-called french paradox refers to a relatively low risk of cardiovascular disease compared to the nutritional intake of cholesterol and saturated fats (Fig. 11). It has been suggested that the polyphenols present in red wine and vegetables protect against oxidation of lipoprotein particles thereby reducing the onset of atherosclerosis.
As we have seen in this chapter ROS generate cellular damage, but we have also powerful mechanisms to prevent this damage and to repair it. However, over a lifetime of metabolism, unrepaired damage accumulates. This is a slightly different theory of ageing, emphasising the wear and tear of cells. Since most oxygen radicals are produced in the respiratory chain, it can be concluded that the more slowly we metabolise, the less oxygen radicals are being produced. In favour of this notion, the average life expectancy is low in small mammals, such as mice. Because of the higher surface-to-weight ratio, smaller animals lose more heat and therefore need to have a faster metabolism to keep up body temperature. Consistently, ectotherm animals typically have a long life expectancy and so have large endotherm animals, such as elephants and humans. In humans it is important to discriminate between healthspan (the number of years experienced with overall good health) and lifespan ( the maximum life expectancy, which is approx. 120 years). Medical advances had a major impact on healthspan in the past 200 years, but not on lifespan.
One way to slow down metabolism is to reduce caloric intake. In support of this notion, lifespan does increase in organisms subjected to reduced caloric intake, but the effects are marginal in larger organisms (Table 2).
|
Organism
|
Lifespan increase
|
Indicator
|
|---|---|---|
|
Yeast
|
3-fold
|
Reduced mutations
|
|
Nematodes
|
2-3-fold
|
Extended mobility
|
|
Flies
|
2-fold
|
Extended mobility
|
|
Mice
|
1.3-1.5-fold
|
Reduced tumor incidence; reduced fatty liver, improved insulin sensitivity |
|
Monkeys
|
None
|
protection against obesity, diabetes, cancer, cardiovascular disease |
|
Humans
|
Not determined
|
protection against obesity, diabetes, cardiovascular disease |
To achieve the effects of caloric restriction, nutrient intake must be reduced significantly, for instance by 30%. This is not attractive to most people. The questions arises whether there are nutrients or drugs that could achieve similar effects as caloric restriction? Analysis of the polyphenol composition of red wine has singled out resveratrol as a particularly powerful component. These compounds activate a group of proteins called sirtuins. Sirtuins have a strong long-term effect on metabolism through AMP-kinase a protein kinase activated by elevated AMP levels, which occur during exercise. We will investigate this pathway in more detail in Chapter 18. There are still many open questions in this area of research. Epidemiological studies (for example the In CHIANTI study) have not found a correlation between resveratrol levels and cardiovascular disease. Also supplementation studies with antioxidants have shown little effect.
- Question
- Answer
What are the three main functions of the pentose-phosphate pathway?
1) to provide NADPH for biosynthetic purposes; 2) to provide NADPH for the removal of reactive oxygen species; 3) to provide ribose-5-phosphate for nucleotide biosynthesis
- Question
- Answer
What is the principal mechanism underlying the generation of oxygen radicals?
Electron transfer onto and from coenzyme Q (or Ubiquinone) generates a semi-quinone, which can transfer an electron to oxygen or to reactive oxygen species already generated elsewhere.
- Question
- Answer
Describe two mechanisms by which ROS can create cellular damage.
Oxygen radicals can cause lipid peroxidation. Extensive lipid peroxidation causes membrane leakage, cell and organellar swelling. Oxygen radicals can cause modification of nucleobases, particularly formation hydroxyguanine. This can result in mispairing during DNA replication.
- Question
- Answer
Why does vitamin E protect against heart disease?
Vitamin E removes the unpaired electron from lipidperoxyl-radicals. While it does not restore the original lipid, a chain reaction is terminated.
- Question
- Answer
Briefly outline the mitochondrial theory of ageing.
Ageing is caused by slow deterioration of mitochondrial function in all cells. This is caused by oxygen radicals and mistakes during mitochondrial DNA replication. This causes miss-assembly or malfunction of mitochondrial respiratory chain complexes.
- Oxygen radicals are continuously produced by the respiratory chain.
- There are a variety of defence mechanisms against ROS, particularly catalase, superoxide dismutase and glutathione peroxidase and vitamins.
- Glutathione peroxidase requires NADPH, which is provided by the pentose-phosphate pathway.
- Radicals are important in neutrophils and in signalling.
- Oxygen radicals may damage mitochondrial DNA, protein and lipids resulting in ageing.
- Antioxidants, however, have failed to provide significant protection against ageing.
- Calorie restriction and sirtuin activators may protect against some signs of ageing.
- It is unclear whether they affect the maximum age.
- Fig. 1
- Fig. 2 By the author using ChemDraw
- Fig. 3 By the author using ChemDraw
- Fig. 4 Dare AJ, Bolton EA, Pettigrew GJ, Bradley JA, Saeb-Parsy K, Murphy MP. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015 Aug;5:163-8. doi: 10.1016/j.redox.2015.04.008. Central PMCID: PMC4427662. Available via creative commons license
- Fig. 5 By the author using ChemDraw
- Fig. 6 By the author using ChemDraw
- Fig. 7 By the author using ChemDraw
- Fig. 8 By the author using ChemDraw
- Fig. 9 By the author using ChemDraw
- Fig. 11 Ferrières J, 2004, The French paradox: lessons for other countries. http://dx.doi.org/10.1136/heart.90.1.107