19 Biochemistry of a growing cell
- Appreciate the building blocks required to make a cell
- Get an overview of biosynthetic pathways
- Understand strategies to target biosynthetic pathways in cancer treatment
- Cancer as a genetic disease
- Metabolic reprogramming in growing cells
- Exploiting metabolic rewiring for diagnosis and treatment
In this book we have followed the metabolism of nutrients, how they generate energy and how they are stored. These are typical functions carried out by differentiated cells that do not grow. In this chapter we want to look at some of the specific modifications of biochemical pathways in growing cells. Much of what we know has come from the study of cancer cells, but even in a healthy body we have populations of growing cells, such as stem cells that produce red blood cells or intestinal epithelial cells. Cells of the immune system can divide rapidly, when activated by antigens. Fibroblasts divide to heal wounds.
When a cell grows there needs to be a trigger to do so. This is typically provided by growth hormones. Growth hormones a released by our own body, for example, to build up muscle mass. Growth hormone signalling is similar to insulin receptor signalling, which you encountered in chapter 8. In fact, insulin stimulates protein synthesis, a requirement for cell growth.
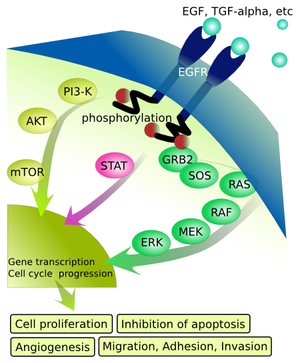
It starts with a receptor tyrosine kinase (EGF receptor in Fig. 1), which autophosphorylates upon binding of the growth factor (Fig. 1). The phosphorylated tyrosine residues are recognised by specific adaptor proteins such GRB2 (Growth factor receptor bound protein 2), which become phosphorylated in the process. This initiates a protein phosphorylation cascade, which finally ends with transcription factors, which become activated, move into the nucleus and initiate transcription of a large number of genes involved in cell proliferation, migration and blood vessel formation etc. Please note that growth factor signalling stimulates protein synthesis as well, via the same pathway as insulin (via PI3-K, AKT and mTOR, Fig. 1). Without a growth hormone, cells remain in their specific tissue or organ and do not divide. One of the breakthrough findings of cancer research was the recognition that cancer is a genetic disease caused by “switch-on” mutations primarily in growth factor signalling pathways, which allows cancer cells to grow in the absence of growth factors. However, cells have significant checkpoints and balances in place to compensate for the occasional mutation in a growth signal transduction pathway. More than one mutation is required to override those checks and balances. These additional mutations are often found in the proteins that carry out the checks and balances. This is one of the reasons why cancers typically develop in older people, when mutations have accumulated over a lifetime. Typically, only a handful of mutations are required in key proteins. For instance, although melanoma cells typically have thousands of mutations caused by UV radiation, only a handful in critical places cause pigmented skin cells to become melanoma cells. In the majority of cancer cells mutations in RAS and RAF, which are central in growth factor signalling (Fig. 1), are found. The accumulation of mutations also explains why cancers can be more frequent in some families. If a critical mutation is already inherited, less additional mutations have to be accumulated.
Random mutations rarely switch a protein “on”, they rather render it non-functional. How can random mutations switch on growth factor signalling? RAS proteins are GTP binding proteins similar to those we have a encountered in our discussion of GPCR signalling (Chapter 12 and Chapter 8). To terminate signalling, G proteins hydrolyse GTP to GDP, which switches the protein “off”. Mutations in RAS change the key catalytical residues required for GTP hydrolysis. The catalytical activity is destroyed, and as a result RAS can no longer switch off! This is also a conundrum for cancer chemotherapy. You can’t block something with a drug that is already non-functional. New approaches try to degrade the whole protein in a cancer cell.
When a cell converts from a differentiated cell into a fast-growing cell it needs a lot of building blocks (Table 1). In the table the macromolecules are listed at the top and the main required building blocks listed underneath. Energy for these pathways is listed separately.
|
Protein
|
DNA/RNA
|
Lipids
|
Energy
|
|---|---|---|---|
|
Amino acids
|
Ribose
|
Acetyl-CoA
|
ATP
|
|
Trace metals, Ca2+, Mg2+, PO4(3-)
|
Aspartate
|
Glycerol
|
NADPH
|
|
Vitamins
|
Glutamine
|
Sphingosine
|
NADH
|
|
Carbohydrates
|
Glycine
|
Choline
|
GTP
|
|
--
|
5,10-Methylene-THF
|
Serine
|
CTP, TTP
|
|
--
|
N10-Formyl-THF
|
Inositol
|
--
|
|
--
|
--
|
Carbohydrates
|
--
|
Some of these building blocks are essential nutrients (like vitamins, trace minerals, essential amino acids) and are acquired through transport. All other building blocks can be synthesised by any normal cell, but the corresponding pathways may be running at a low rate. As we learned earlier, even differentiated cells are in constant turnover and therefore break down protein, RNA and lipids only to reassemble them into the same biomolecules again. A dividing cell, by contrast, has a large net requirement for new building blocks on top of turnover. The first recognition of the high demands for building blocks in cancer cells, in this case 5,10-Methylene-THF (see chapter 15), came from studying lymphoma cells in childhood leukemia (see case study).
How Vegemite helped to treat Leukaemia
Poor undernourished workers in Bombay in the 1920’s were often anaemic, particularly women after giving birth. Lucy Wills a young British physician found that vegemite cured this form of anaemia. The “Wills factor” in vegemite turned out to be folate (see chapter 15). Sidney Farber a cancer physician at Harvard University thought that folate could be used to normalise the blood cell status in children with Leukemia, because it also normalised the blood cell development in anaemia.
He gave folate to children with leukaemia only to find that it actually worsened the leukaemia. He quickly stopped the treatment and revised his idea. May be “antifolates” could cure cancer. The chemist Yellapragada Subbaro (Yella) had supplied Farber with folate. However, he never got recognition at Harvard for his many other discoveries and went to the pharmaceutical industry. There he synthesised a number of folate derivatives some of which functioned as “antifolates”. In 1947 Farber received the first antifolates from Yella. Robert Sandler, a two year old child was admitted at Children’s Hospital in 1947. His leukaemia got worse and worse, causing bone fractures as the cancer moved through the bone and caused indescribable pain. Without any permission, Faber tried two new antifolates that he had received. One didn’t work, but the second, aminopterin, almost worked miracles. The white blood cell count returned to normal and by January 1948 Robert Sandler could walk on his own and became hungry again. The swelling of spleen and liver disappeared. It was the first time that a drug could cure cancer and bring hope to children with Leukemia. Source: Siddhartha Mukkerjee, The Emperor of all Maladies
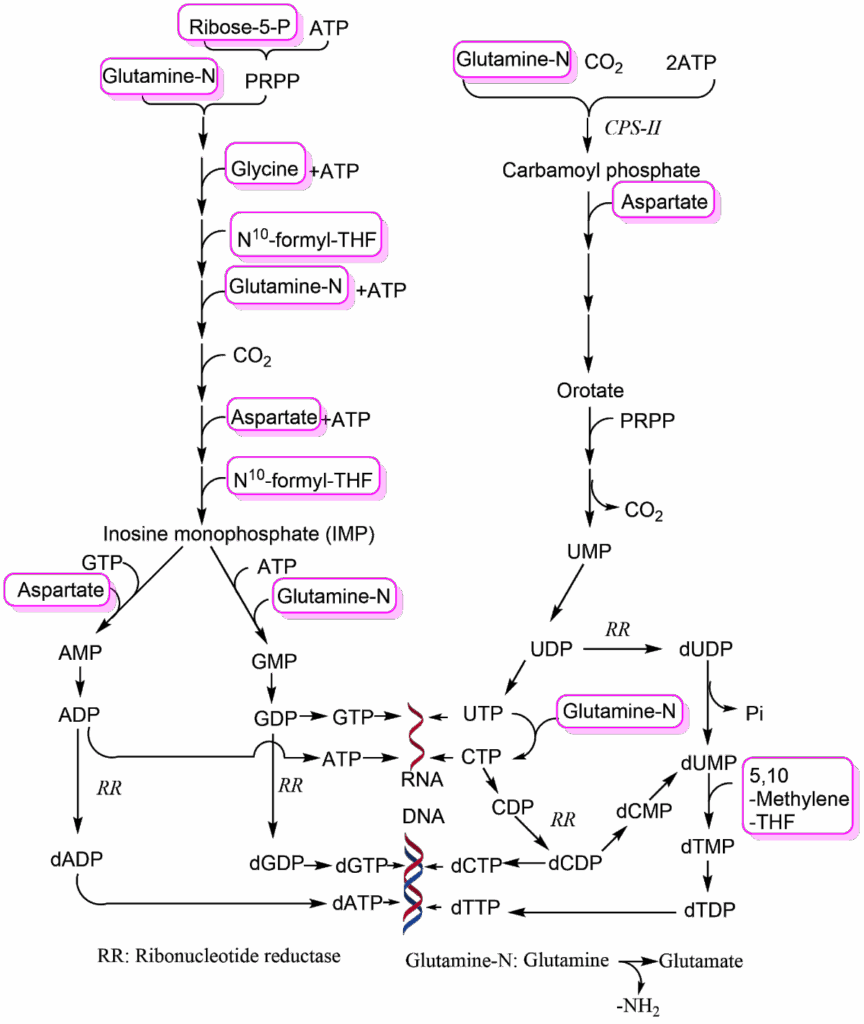
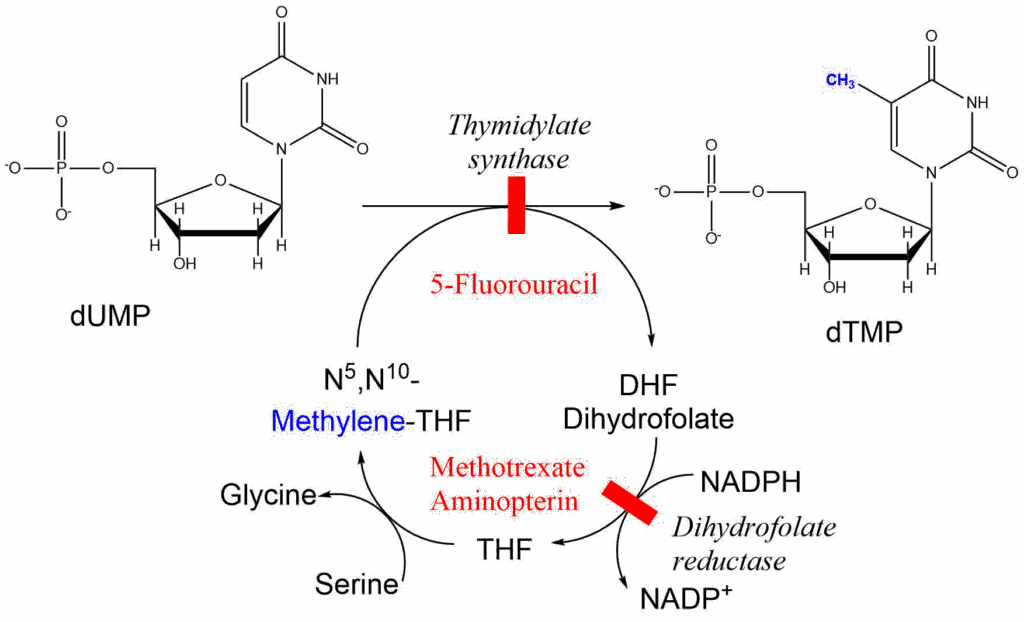
Aminopterin has been superseded by methotrexate; both are inhibitors of dihydrolofate reductase which converts dihydrofolate (DHF) back to tetrahydrofolate (THF). The C1-compound is recharged through serine conversion into glycine. Please note that the methylene-group when attached to THF has a different oxidation state than the methyl-group attached to thymine. As a result, THF is converted to DHF and needs to be reduced by NADPH back to THF. Thus, DNA synthesis also requires significant amounts of NADPH, which together with ribose-5-phosphate (Fig. 3) can be provided by the pentose-phosphate pathway (PPP). As a result, the PPP is more active in a growing cell than in a differentiated cell (see Fig. 5 for compounds that reduce PPP activity).
Viruses use the cellular machinery for their own purposes to replicate and produce many virus particles, each containing RNA or DNA with their genomic information. Human cells use metabolic depletion of deoxynucleotides as an antiviral strategy.
Goldstone, David C., et al. “HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase.” Nature 480.7377 (2011): 379-382.
The high activity of the PPP would perhaps suggest that glycolysis is slower in cancer cells, but the opposite is the case. This is called the Warburg effect after its discoverer Otto Warburg. It is caused by a suppression of pyruvate dehydrogenase activity in cancer cells (Fig. 5). Thus, pyruvate does not get fully oxidised, and instead is converted into lactate to recycle NADH. Remember that the yield of ATP in glycolysis is much lower (2 ATP/glucose) than that achieved by mitochondrial respiration (36 ATP/glucose) and as a result metabolic flow through glycolysis increases significantly to provide enough ATP. This metabolic adaptation is typically switched on by lack of oxygen (hypoxia). This can occur where limited number of blood vessels restrict the flow of oxygen to cells, such as in a solid tumour where blood vessel growth has not yet occurred. Remarkably, tumours can only grow to a few millimetres in size in the absence of blood vessels. However, cancer cells still show the Warburg effect even in the presence of oxygen.
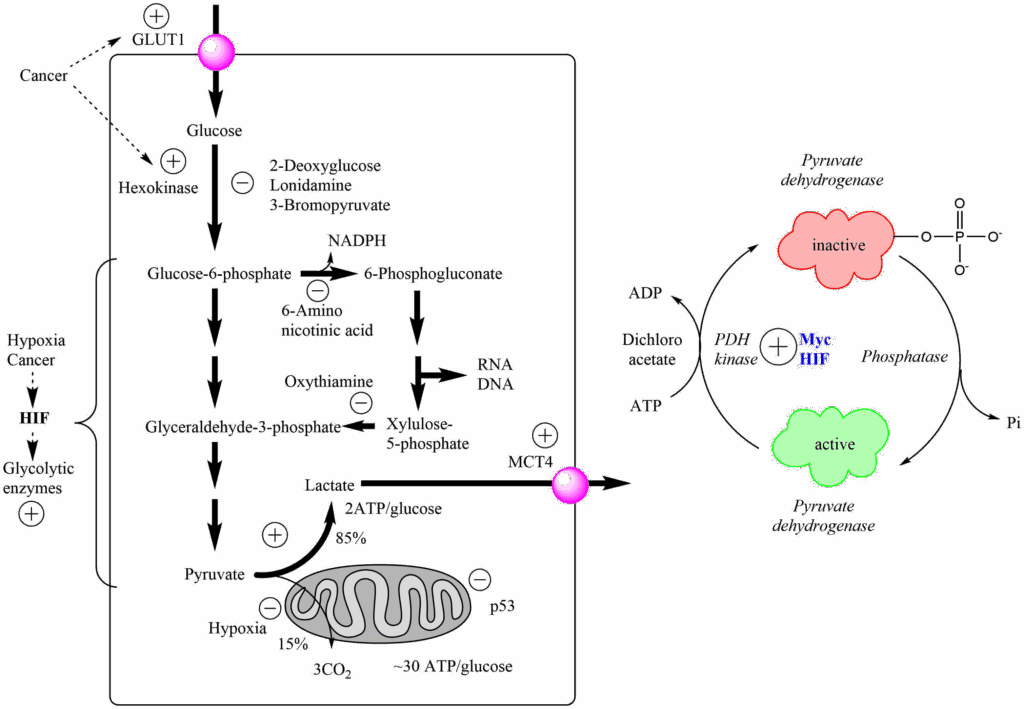
This metabolic program (the Warburg effect) is switched on in cancer cells even in the presence of oxygen, due to increased expression of the transcription factors Hypoxia induced factor (HIF) and the myc oncogene, which in turn increase expression of pyruvate dehydrogenase kinase (PDH-kinase). PDH-kinase phosphorylates and inactivates pyruvate dehydrogenase (Fig. 5). Some cancer drug candidates are listed in Figure 5 that target different steps of glycolysis or the PPP, but none of these has been very successful in clinical trials. However, the large flux through glycolysis is used diagnostically to detect cancers and to monitor treatments (see case study below). In this case 2-[18F]Fluoro-2-deoxyglucose (FDG) is used as a positron emission tracer (PET) to detect tissues with high glycolytic activity. FDG is converted by hexokinase into 2-[18F]Fluoro-2-deoxyglucose-6-phosphate and thus trapped inside the cell (see chapter 7) but cannot be metabolized further.
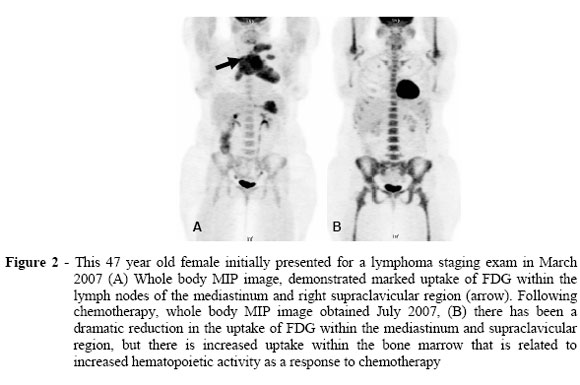
Fig. 6 shows accumulation of [18F]Fluoro-2-deoxyglucose (FDG) in 47-year-old female before (A) and after (B) chemotherapy. Please note the strong signal in heart and brain, which have physiologically high rates of glycolysis. Other dark areas are cancer tissue
CARTER, Kevin R. and KOTLYAROV, Eduard. Common causes of false positive F18 FDG PET/CT scans in oncology. Braz. arch. biol. technol. [online]. Available under Creative commons license.
Lipid biosynthesis is highly active in cancer cells because new membranes are required when cells divide. However, cancer cells also use significant amounts of fatty acids and lipoproteins from the circulation to satisfy demands for the synthesis of new membranes. The pathways involved are the very same pathways we have discussed in chapter 12 and 13.
We now want to return to the large demand of cancer cells for glutamine and aspartate, which is caused by their use for the synthesis of nucleotides and also for some other pathways. Both are non-essential amino acids, but surprisingly cancer cells often depend on glutamine or asparagine for growth. In particular “glutamine addiction” has been noted in many cancer cells. Thus, a non-essential amino acid becomes a conditionally essential amino acid, because it is used as a building block for several pathways, including the conversion of aspartate to asparagine. When aspartate is synthesised it is derived from the TCA cycle intermediate oxaloacetate. Although cancer cells generate a large amount of ATP from glycolysis, they still rely on the TCA cycle, as well. When oxaloacetate is withdrawn to generate aspartate, the canonical TCA cycle would have less and less capacity to generate energy. In chapter 9 we saw that anaplerotic reactions are used to refill intermediates of the TCA cycle. Tumour cells go one step further and convert the TCA cycle into the linear glutaminolysis pathway (Fig. 7).
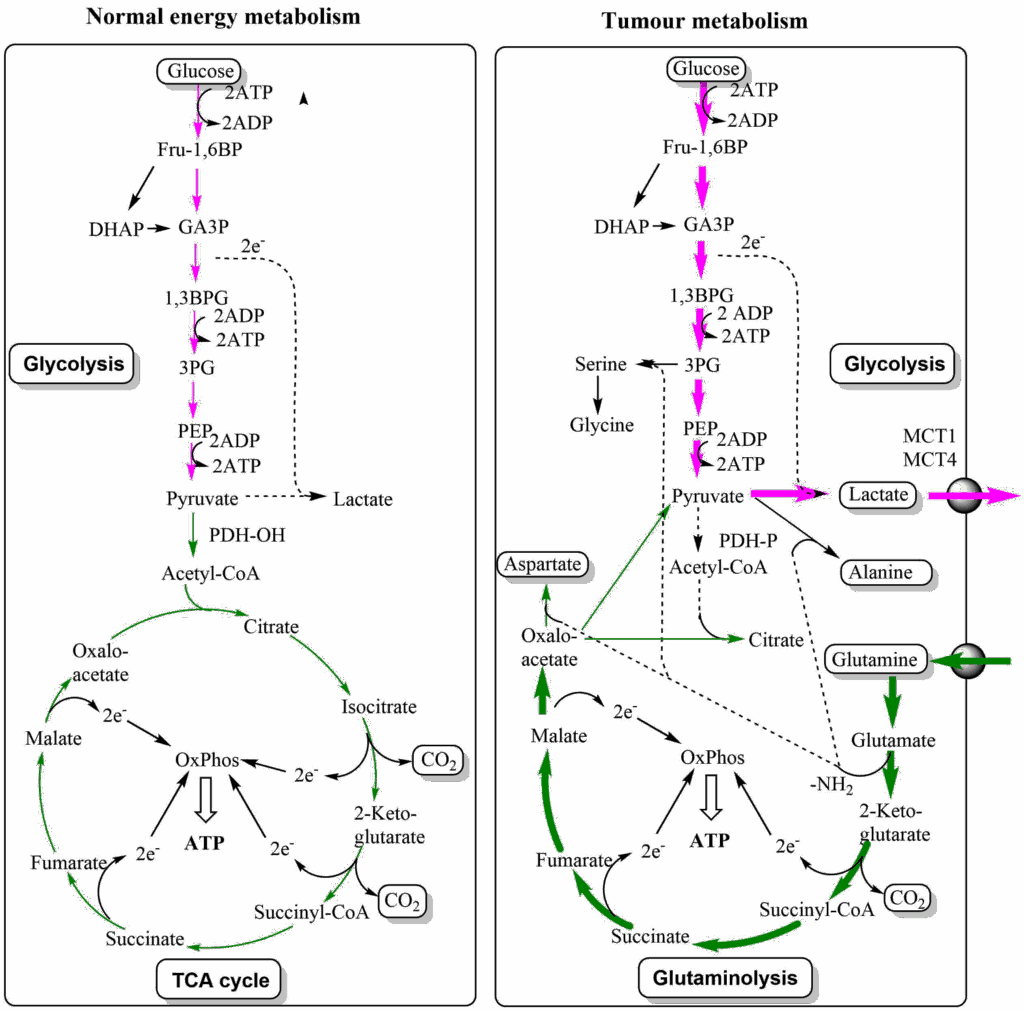
The pathway starts with glutamine, which is converted to glutamate and then to 2-ketoglutarate, joining the TCA cycle. The cycle is followed up to oxaloacetate, which is converted into aspartate. Please note that this also recycles an amino-group, which was liberated by the transaminase reaction converting glutamate to 2-ketoglutarate. Glutamine is the most abundant amino acid in blood, and thus easily obtainable by growing cells. The pathway generates ATP through respiration and can be extended to generate citrate for fatty acid biosynthesis. The Warburg effect and TCA cycle remodelling are synergistic changes allowing cancer cells to produce large amounts of building blocks without compromising energy production.
We saw earlier in this chapter that C1-compounds are required for nucleotide biosynthesis. C1 compounds can be generated through a branch in glycolysis, converting 3-phosphoglycerate to phosphopyruvate (Fig. 8).
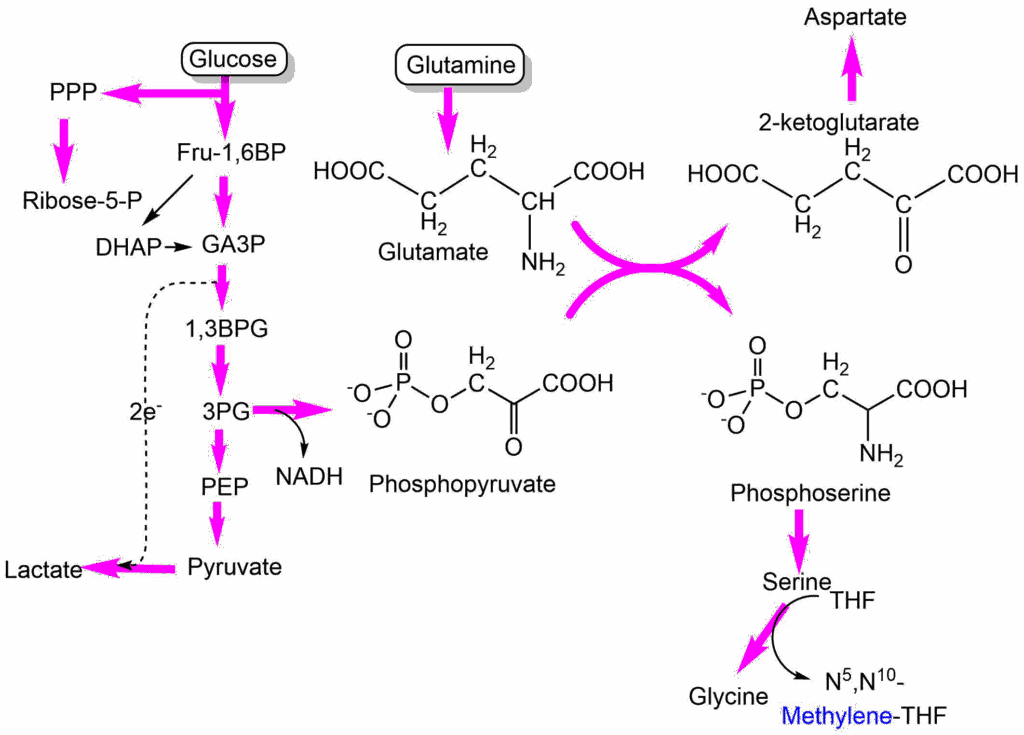
Phosphopyruvate is an acceptor for amino-groups allowing transamination of glutamate to 2-ketoglutarate, while it becomes phosphoserine. Phosphoserine is dephosphorylated to serine, which can be converted to glycine to generate N5,N10-methylene-THF. The 2-ketoglutarate can go into the glutaminolysis (TCA cycle) pathway to produce aspartate.
The metabolic adaptation of cancer cells include the overexpression of amino acid transporters and specific lactate transporters. Even specific oncometabolites have been discovered that do not occur in untransformed cells or only in trace amounts. For instance, mutated from of isocitrate dehydrogenase convert their normal product α-ketoglutarate to 2-hydroxyglutarate, which influence epigenetic mechanisms. It shows that a growing cell has quite different metabolic demands compared to a differentiated cell and that genetic programs exist that can be switched on to support cell growth and division. As the example of antifolates demonstrate, these adaptations can make cancer cells more vulnerable to certain inhibitors than differentiated cells.
- Question
- Answer
What are the main uses of glutamine in cancer cells?
Glutamine several times donates its nitrogen for the synthesis of nucleobases. It is also the precursor to generate aspartate, which is also required for nucleotide biosynthesis. Glutamine generates energy by feeding into the TCA cycle and also produces other amino acids and citrate for fatty acid biosynthesis through the glutaminolysis pathway.
- Question
- Answer
What is the rationale for the use antifolates in cancer chemotherapy?
Conversion of UMP to TMP for DNA biosynthesis requires attachment of a methyl-group. The methyl group is transferred from methylene-tetrahydrofolate, which needs to be recharged to donate the next methyl group. The recharging is blocked by antifolates.
- Question
- Answer
Describe two reactions in cancer metabolism where transaminase/aminotransferase reactions are critical.
The conversion of glutamate to 2-oxoglutarate is critical as an anaplerotic reaction of the TCA cycle. For an ongoing reaction the amino-group must be deposited onto keto acids. This could be oxaloacetate (forming aspartate), in which case no anaplerosis occurs; or phosphopyruvate to form phosphoserine, which can be used to replenish C1 metabolism.
- Question
- Answer
Why can 2-Fluoro-2-Deoxyglucose (FDG) be used to detect cancer cells?
Many cancer cells display the “Warburg effect” an up-regulation of glycolysis to produce lactate instead of using mitochondrial respiration. The increased flux through glycolysis captures FDG, which becomes phosphorylated and trapped inside the cancer cell.
- Question
- Answer
The case study (Fig. 6) notes high levels of glycolysis in bone marrow after chemotherapy. Why is this the case?
Chemotherapy has destroyed many stem cells that generate erythrocytes and immune cells. After chemotherapy these stem cells become active to rebuild the immune system and generate red blood cells. Stem cells have a similar metabolism as cancer cells and show a Warburg effect when dividing fast.
- Cancer arises due to mutation in growth factor signalling pathways.
- Rapid growth of cells is accompanied by metabolic remodelling.
- Synthesis of nucleotides requires large amounts of C1 compounds, which are transferred by tetrahydrofolate. This requirement has been targeted by antifolate therapy.
- The Warburg effect refers to a reduction of mitochondrial respiration in cancer cells. Flux through glycolysis is enhanced to provide sufficient ATP. This can be used diagnostically through imaging of fluorodeoxyglucose.
- Remodelling of the TCA cycle into the linear glutaminolysis pathway, allows generation of aspartate for nucleotide biosynthesis and other building blocks.
- Glucose metabolism includes the PPP to produce ribose-5-phosphate and generation of serine as a precursor for C1 metabolism.
- Fig. 1 Eikuch [Public domain], via Wikimedia Commons
- Fig. 2 Unknown photographer/artist [Public domain], via Wikimedia Commons
- Fig. 3 By the author using ChemDraw
- Fig. 4 By the author using ChemDraw
- Fig. 5 By the author using ChemDraw
- Fig. 6 CARTER, Kevin R. and KOTLYAROV, Eduard. Common causes of false positive F18FDG PET/CT scans in oncology. Braz. arch. biol. technol. [online]. Available under Creative commons license.
- Fig. 7 By the author using ChemDraw
- Fig. 8 By the author using ChemDraw