4 Protein structure and function
- To understand the relevance of protein structure for protein function.
- To understand the molecular interactions that determine the shape of proteins.
- To appreciate the dynamic nature of proteins and their denaturation by heat and acid
- Levels of protein structure
- Interactions that stabilise protein structure
- Active site
- Induced fit
Although this lecture series focuses on nutrients and their metabolism, we will come across proteins in many forms, such as enzymes, receptors, transporters, peptide hormones etc.
A basic understanding of proteins is important to understand nutrient metabolism. Let us have a look at some proteins (Fig. 1) and watch them in action (video below).
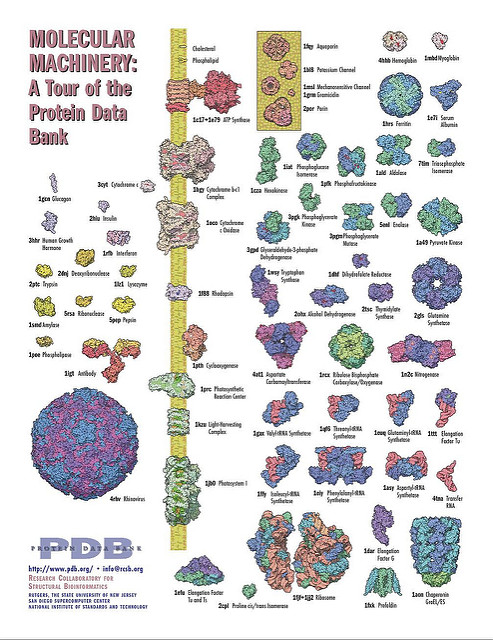
Several important concepts of protein structure can be appreciated just by looking carefully at Fig. 1. There are simple proteins that contain only one protein chain (a single colour, encoded by one gene). Others are complex and are comprised of several chains (different colours and encoded by several genes). Ribosomes (at the bottom of Fig. 1) are even a mix of RNA and proteins. Most proteins have a globular appearance, which suggests that a compact structure is a favourable arrangement.
All proteins are made up of the 20 amino acids, which you encountered in chapter 1. Why are proteins not unwinding into a long thread of amino acids? The linear arrangement of amino acids is held together by covalent bonds, which are very strong. Even when boiled these bonds do not break. The string of amino acids making up a protein is called the primary structure.
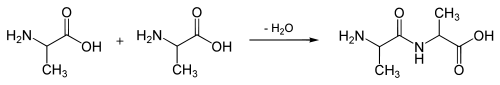
The bond between two amino acids is called the peptide bond and is generated by elimination of water when amine of one amino acid reacts with the carboxyl-goup of another amino acid. Breakdown of peptides thus occurs by hydrolysis. Inspection of any peptide reveals atoms that are polarised. The carbonyl-oxygen (C=O) is partially negative and the hydrogen associated with the nitrogen is partially positive. Hydrogen bonds (dotted) can form between these groups: =N-H…O=C if they are in close proximity.
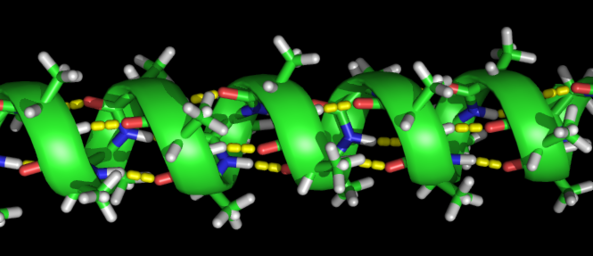
When the peptide is threaded like a helix and one turn takes about 4 amino acids, the carbonyl-oxygen (C=O) can form a hydrogen bond (yellow in Fig. 3) with a Nitrogen-Hydrogen (N-H) of the residue four positions further on or behind. This structure is very stable, because the amino acids do not need to find other partners to interact with. As a result, most proteins have a good proportion of amino acids forming alpha-helices. Important to note: In an alpha-helix side chains point away from the centre of the helix (Fig. 3) avoiding steric problems. Another structure that proteins can form readily is the beta-sheet (Fig. 4).
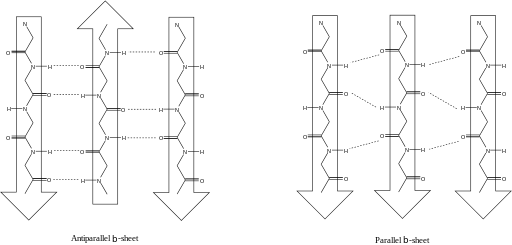
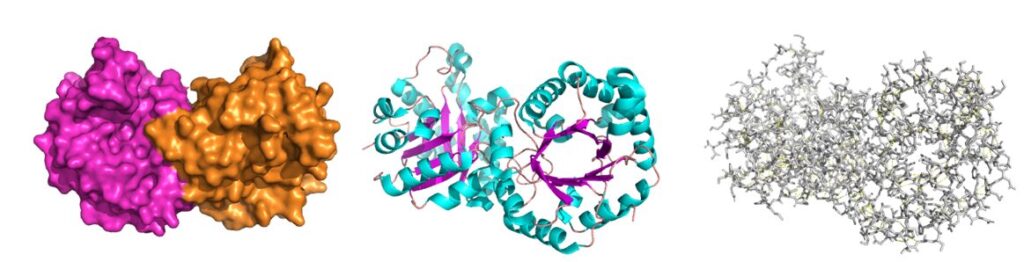
The left presentation shows the surface of the protein as you would see it at very high magnification. Highlighted are the two chains of the protein forming a dimer of identical subunits. An arrangement of several peptide chains forming a functional protein is called the quarternary structure. In the center panel the secondary structures are highlighted. Helices are in blue, beta sheets in magenta and turns in red. On the right the primary structure is depicted, which is rather confusing. The yellow hue comes from highlighting hydrogen bonds. While you cannot see individual ones, it illustrates how many there are to hold the proteins together. Hydrogen bonds are depicted in more detail in Fig. 3 and 4. Hydrogen bonds are one reason that proteins do not unwind spontaneously. However, there are more interactions that hold proteins together (Fig. 6). They are called weak interactions, because the bond energy is much lower than that of a covalent bond.
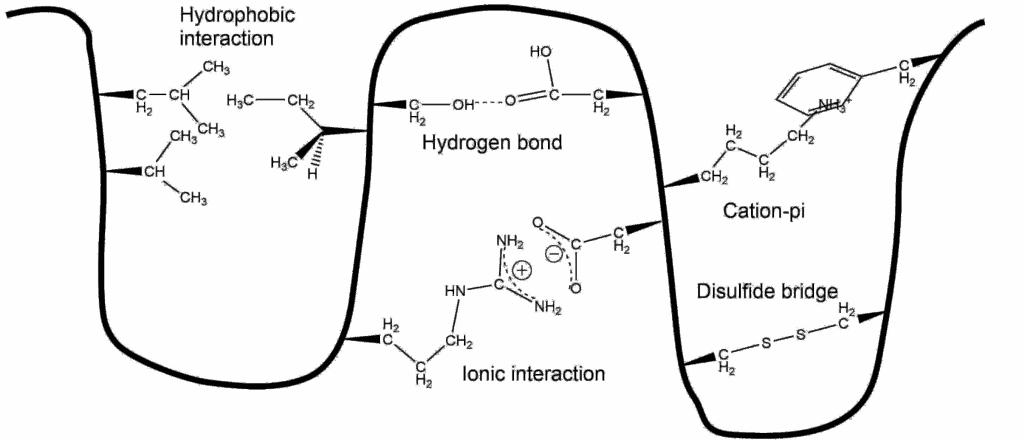
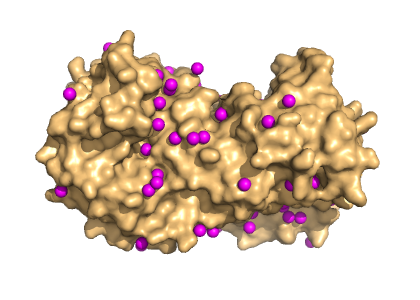
You already appreciate the role of hydrogen bonds in holding secondary structures together, but they also are important to stabilise tertiary and quarternary structures. Another important interaction is the hydrophobic effect. It is based on the gain of entropy when water molecules are released from the core of the protein. Non-polar sidechains are typically found in the core of a protein, which cannot form hydrogen bonds with water molecules. If water molecules were present in the core they would have to from concatenated structures of hydrogen-bonded water molecules with little mobility, which is a state of low entropy. Although fixed water molecules are rarely found inside proteins, high resolution structures show quite a few water molecules tightly bound to the surface of proteins (Fig. 7).
In addition, charged sidechains can form ionic bonds. Hydrogen bonds can form between polar hydrogen-containing bonds of carbon, nitrogen and oxygen to other atoms with a free electron pair within a protein or between protein subunits. Cation-pi interactions form because aromatic rings are electron-rich structures that can interact with electron poor positively charged sidechains. These weak interactions can be broken by heating or addition of acid, in which case the protein denatures (see video below). Denatured proteins look white and curdled. Denaturation of protein occurs in the acid environment of the stomach and facilitates the access of proteases to hydrolyse peptide bonds.
You have now a good idea how proteins are held together. One of the main functions of the three-dimensional protein structure is to bring critical residues together that form the active site of an enzyme.
Why would heat and acid treatment cause denaturation of protein?
Covalent bonds are not broken by heat, but non-covalent bonds that hold together a protein structure are weak bonds that can be broken by heating. Acid treatment changes the ionisation of side-chains and thus breaks hydrogen bonds
In Fig. 8 you can see key residues (with reactive atoms in blue and red) forming the active site of triosephosphate isomerase. They emanate from different helices and sheets of the protein. The substrate (purple) is located in the centre of the active site.
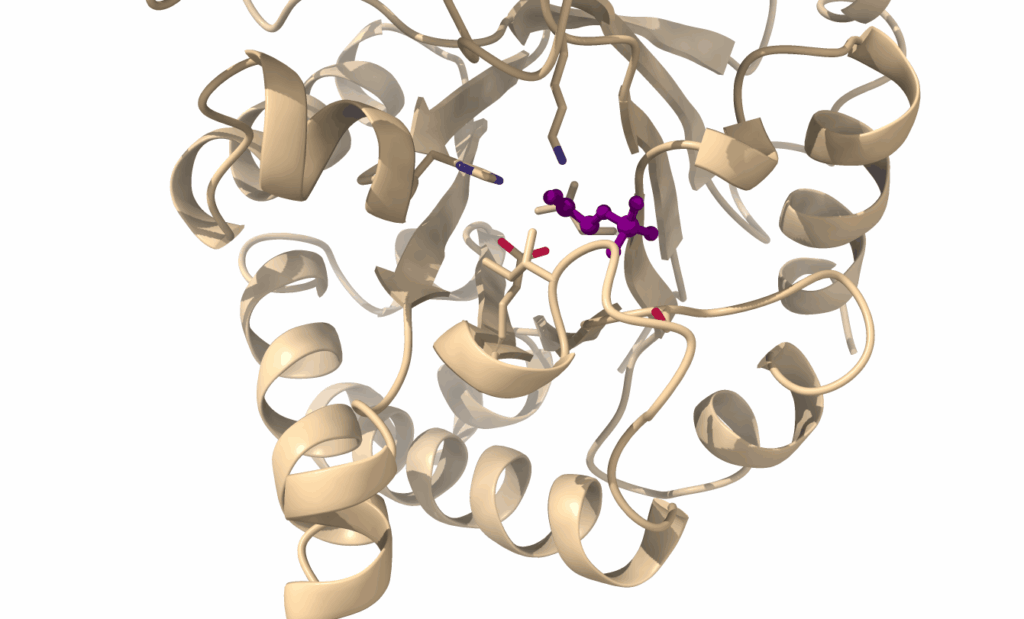
We often imagine proteins as rigid and the substrates colliding with them. When we cover enzymatic catalysis you will see that interactions between the substrate and the enzyme are critical to achieve a substrate conformation which facilitates a chemical reaction (Chapter 5). This often includes some conformational change of the protein, as well. This is particular obvious in the case of hexokinase, which encloses glucose during catalysis. This mechanism is called induced fit (Fig. 9). Induced fit is particularly important in the catalysis of membrane transport (Chapter 6).
- The primary structure of a proteins is the linear chain of amino acids connected by peptide bonds
- Secondary structures are typical structural elements that are readily formed by a chain of amino acids and are found in many proteins. Typical secondary structures are alpha-helix, beta-sheet and turns.
- The tertiary structure is the unique shape adopted by a full-length protein chain.
- The quarternary structure refers to a complex of two or more protein chains making up a larger protein complex.
- Secondary, tertiary and quaternary structures are held together by different molecular interactions, such as hydrogen bonds, hydrophobic effect, ionic interactions, cation-pi interactions and disulfide bridges.
- While covalent bonds cannot be broken by heat and treatment with acid, secondary, tertiary and quarternary structures are denatured, resulting in curdling of the protein. This is an important part of protein digestion in the stomach.
- The precise tertiary structure is important to align all residues that are critical for catalysis.
- Ligands and protein have dynamic interactions, which changes the structure of the ligand, but also changes the structure of the protein.
Looking for a quick review of what you learned in this chapter, watch the video below.
Provide an explanation for each level of protein structure
- Primary structure
- Secondary structure
- Tertiary Structure
- Quarternary structure
- The sequence of amino acids in a protein
- Regular stable structures formed by a string of amino acids
- The overall folding pattern of a protein
- The arrangements of multiple subunits in a protein complex
Which is not a relevant interaction that stabilises the three-dimensional structure of proteins?
- Hydrophobic interactions
- Covalent bonds
- Electrostatic interactions (charge-charge)
- Ester bonds
- Hydrogen bonds
Answer
Ester Bonds
“Lock and Key” and “Hand and Glove” are often used analogies to describe the fit between a substrate and its enzyme. Which one is correct or are they both?
“Lock and key” emphasises the tight fit between substrate and its active site. “Hand and Glove” emphasises the dynamics and flexibility (induced fit) between enzyme and substrate. Most enzymes are quite specific but also are dynamic when binding a substrate. As a result, both analogies have their merit.
alpha-Helices are often embedded in a protein so that one side faces the surface and the other side faces the interior. Such helices are termed amphiphilic because the side facing the surface is hydrophilic and the side facing the interior is hydrophobic. A simple way to decide whether a sequence of amino acids might form an amphipathic helix is to arrange the amino acids as a helix-wheel projection. In this projection the helix is viewed from the top and one turn takes a bit more than 4 amino acids. Investigate the 3 peptide sequences below (written in one letter code) and decide which peptide might form an amphiphilic helix. The Mnemonic FAMILY VW will help you recognising hydrohobic amino acids.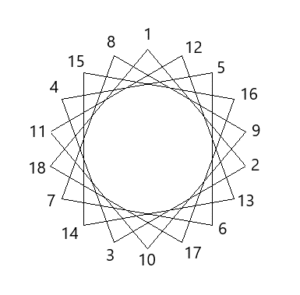
- SLIKSVIEMVDEWFRTFL
- FLIRRKVLVFVRLTRILS
- RLFRSRVLKIAVIRFLLI
SLIKSVIEMVDEWFRTFL
- Fig. 1 Protein Data Bank (https://www.rcsb.org/)
- Fig. 2 Yikrazuul [Public domain], from Wikimedia Commons
- Fig. 3 en:User:Bikadi [GFDL (http://www.gnu.org/copyleft/fdl.html), CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/) or CC BY-SA 2.5 (https://creativecommons.org/licenses/by-sa/2.5)]
- Fig. 4 Mysterioso [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], from Wikimedia Commons
- Fig. 5 By the author using Pymol
- Fig. 6 By the author using ChemDraw
- Fig. 7 By the author using Pymol
- Fig. 8 By the author using ChimeraX
- Fig. 9 By the author using Pymol