6 Membranes and Nutrient Transport
- Understand the fluid nature of a membrane
- Understand how hydrophilic molecules can cross a lipid bilayer
- Appreciate the similarity between enzymes and transporters
- Discriminate between different modes of transport
- Fluid mosaic model and its evolution
- Transporters and channels
- Alternating access
- Primary and secondary active transport
The amphiphilic nature of phospholipids (Chapter 1) results in the spontaneous formation of a bilayer, because this allows hydrophilic headgroups to interact with water, while hydrophobic side-chains are hidden from water. A very influential model for our understanding of membrane structure has been the fluid mosaic model developed by Sanger and Nicholson (Fig. 1).
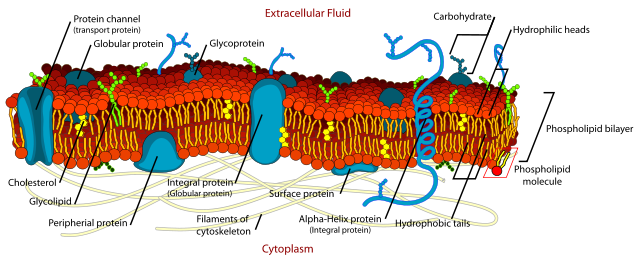
The model proposes a bilayer of phospholipids forming a membrane. Membranes are not rigid, rather behaving as an oily fluid. The bilayer of phospholipids can bend and change shape. The contents of a membrane, lipids and membrane proteins can float in the two dimensions of the membrane. Membrane proteins typically stretch all the way through the membrane bilayer (called integral membrane protein). Originally peripheral membrane proteins were proposed that only stretch through a monolayer (also depicted in Fig. 1), however this arrangement is not stable. It appears that some proteins are rather attached to the surface of the membrane and distort the local phospholipids. Membrane proteins can also be tethered to the cytoskeleton, thereby restricting their mobility. Lipid bilayers are comprised of different ratios of glycerolipids, sphingolipids and cholesterol (Chapter 1). The lipid composition of the two leaflets is different. For instance, phosphatidylserine is only found in the inner leaflet, exposure to the outside is a signal in processes such as cell death and blood coagulation. Glycolipids are only found in the outer leaflet.
You learned earlier that lipid membranes are impermeable to hydrophilic molecules, which includes most metabolites that we will encounter in this book. A notable exception is butyrate, a short-chain fatty acid produced in large quantities by the intestinal microflora from fibre. At the acidic pH prevailing at the surface of the intestine (pH 5.0) a significant fraction of the butyrate is protonated (butyric acid). This molecule can diffuse through the lipid bilayer without facilitation by a protein. Another metabolite that is likely to permeate through membranes to some extent without aid is cholesterol. Water molecules can cross the bilayer to some extent, but larger flows require a dedicated pore called an aquaporin.
All other metabolites require a membrane transporter to cross a membrane. As a result, some metabolites, which do not have a dedicated transporter, are not thought to cross the membrane at all, but precursors for these molecules do. Examples of this are NADH and Acetyl-CoA.
In chapter 5 you learned about enzymes and how they catalyse chemical reactions. Membrane transporters are enzymes that catalyse (facilitate) movement of molecules through a protein pore.
Watch this video (only allowed by link) to get some idea how molecules move through protein pores.
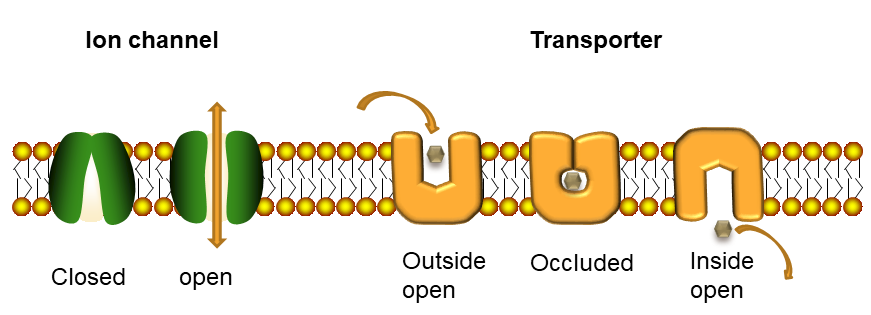
First you will see an ion moving through a channel and then a metabolite moving through a transporter together with an ion. There is a fundamental difference between a channel and a transporter (also Fig. 2). A channel forms a continuous pore through the membrane. Channels can close altogether, but once open they are accessible from both sides and an ion can diffuse all the way through the membrane. A transporter, by contrast, never forms a continuous pore. Transporters have three conformations, namely an outside open state, an occluded state and an inside open state. As a result, a transporter is more like an enzyme undergoing induced fit.
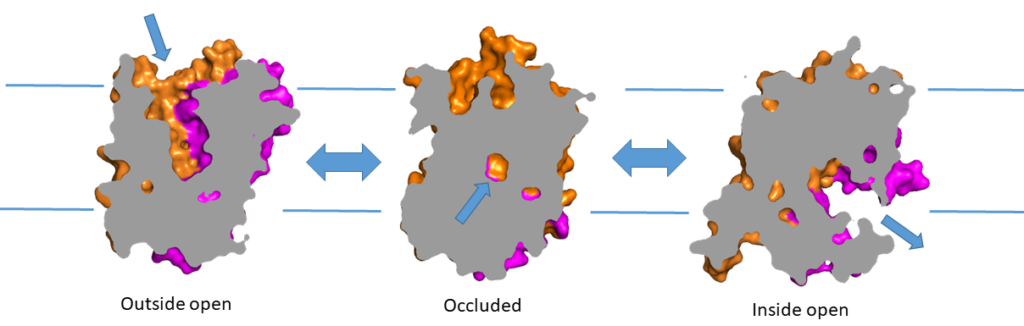
These schemes are supported by high resolution structures of transporters in different conformations (Fig. 3). As an example the bacterial leucine transporter LeuT is shown.
In this book we will not look at ion channels, because we are mainly interested in metabolism and nutrition. As a general rule, translocation of metabolites through membranes is mediated by transporters, while channels rather mediate the passage of ions. The proteinacious pore that is integral to a transporter can be lined by hydrophilic residues, which allow passage of hydrophilic molecules. In the active centre of a transporter, called substrate binding site, residues will bind tightly to the substrate only if the protein encases the substrate. This will prevent further access to the binding site from the outside, the transporter changes conformation to the occluded state. Small thermal movements of the protein now allow further transitioning to the inside open conformation releasing the substrate. This mechanism prevents the accidental transition if ions through the transporter. This mode of operation is also called alternating access, because substrate can alternately bind on the outside or inside, but not on both sides at the same time. Transporters are classified as primary active and secondary active (Fig. 4).
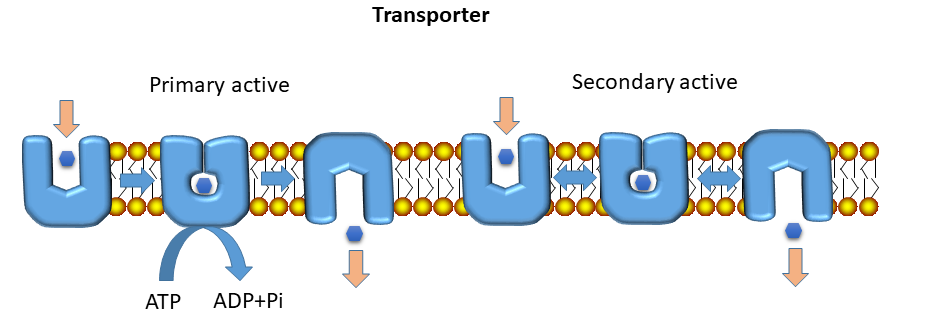
Primary active transporters are driven by ATP hydrolysis. This generates a large amount of energy, the transport is essentially unidirectional (blue arrows in Fig. 3, an animation of primary transport can be found in chapter 3). Secondary active transporters make use of ion and substrate gradients that are established by primary active transporters (explaining secondary). Secondary transporters come in different varieties, such as uniporter, symporter or antiporter (Fig. 5).
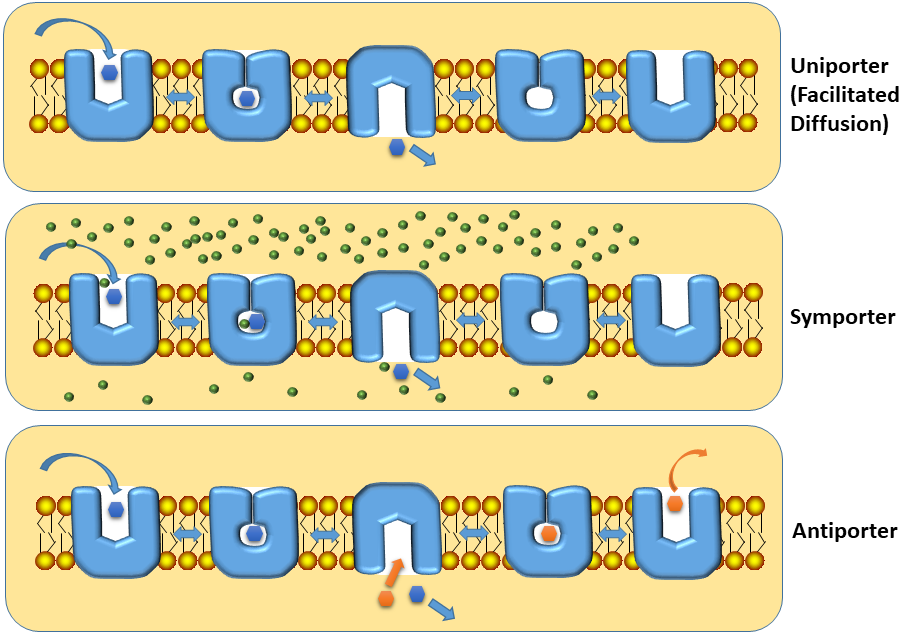
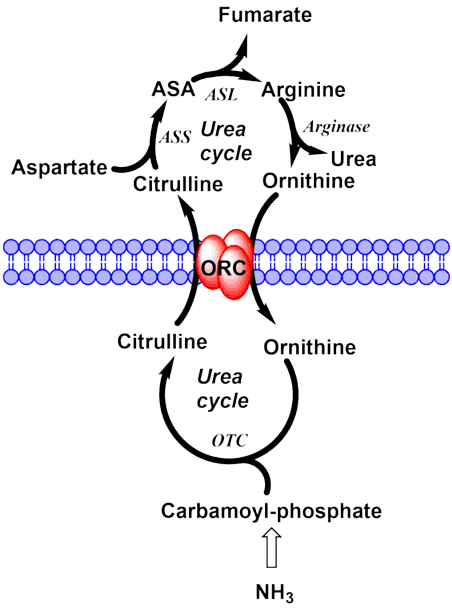
The catalytic cycles of the different types of secondary active transporters are shown in Fig. 5. The uniporter mediates facilitated diffusion that means transport follows the gradient of the substrate. This is the typical mechanism how glucose enters cells in our body, with the exception of epithelial cells. Symporters are used to accumulate substrates within a cell. They make use of ion gradients to give a favourable direction to the transport process. Typically, the concentration of Na+ ions is about tenfold higher in blood (150 mM) than in the cytosol (15 mM). By tying (coupling) the movement of Na+ together with that of a metabolite, the metabolite will be accumulated in the cytosol. Antiporters are often used in mitochondria, where substrate 1 enters and substrate 2 (often a product of substrate 1) is released. An example is the urea cycle, which we will discuss in more detail in chapter 15. The urea cycle would not work without the ornithine/citrulline antiporter, which exchanges two of its intermediates between cytosol and mitochondria (Fig. 6).
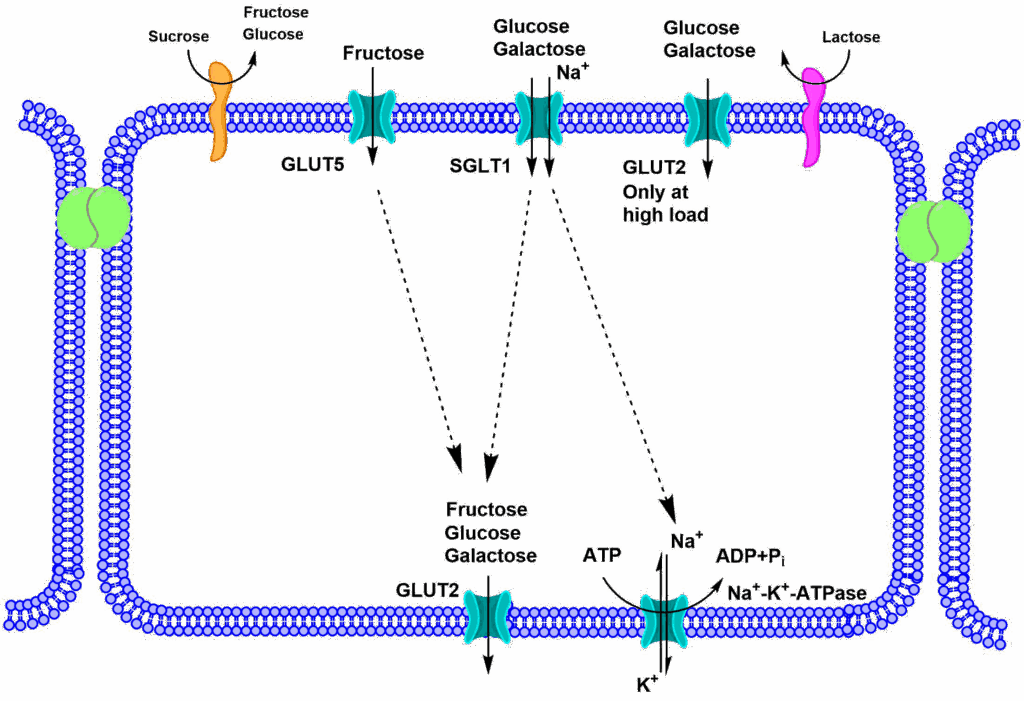
Now you can better appreciate the transfer of sugars through an epithelial cell that we encountered in chapter 5 (Fig. 7). The Na+/K+-ATPase (primary active transport) establishes a Na+ gradient, by pumping Na+ out of the cell. This gradient is used through the symporter SGLT1 to accumulate glucose and galactose inside the cell. Release of all sugars is through facilitated diffusion, via the uniporter GLUT2.
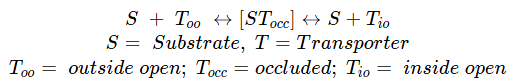
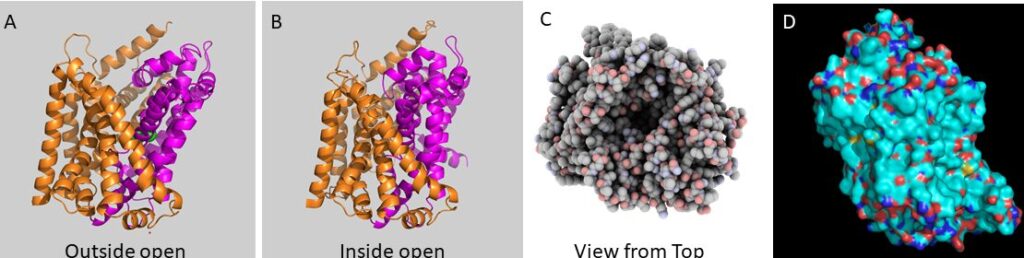
The conformational changes in the bacterial leucine transporter LeuT involve a group of helices (magenta), which perform a rocker-switch like motion against a scaffold domain (orange). Before structures of membrane proteins were available, transmembrane helices could be readily detected by calculating the average hydrophobicity of a stretch of 18-20 amino acids and sliding this window along the primary structure of amino acids. Transmembrane helices showed up as a peak in the plot. For the membrane transporter shown in Fig. 8 the plot shows 10 peaks.
Question
Revisit chapter 1 figure 3 and take into consideration that amino acid side chains point outwards in a helix. Which amino acids would you expect to be enriched in the membrane spanning parts of a transporter protein?
Answer
Amino acids with a non-polar side chain, including aromatic amino acids.
Question
Which amino acids might be suitable to form a narrow turn in a protein structure?
Answer
Amino acids with small side chains, particularly glycine
Question
Let us assume an antiporter that always moves substrate A from in-to-out and substrate B from out-to-in. Is this possible?
Answer
This is not possible, because the antiporter would generate concentration gradients of both substrates without energy input. An exception would be if both substrates had different charges. You can see this in Fig. 6, where ornithine enters the mitochondria because it has a positive charge, while citrulline (0 charge) exits. The mitochondrial membrane has an inside-negative membrane potential.
Pyruvate transfer across the inner mitochondrial membrane is dependent on the proton gradient. The pyruvate carrier transports pyruvate and a proton into the matrix. This accumulates pyruvate inside mitochondria because protons are pumped out of the matrix by the respiratory chain.
How would you classify this transport process?
- Symport
- Secondary active
- Primary active
- Antiport
- Uniport
2. Secondary active
Question
Transporters form a transporter-substrate complex by occluding the substrate. Given what you have learned about the reduction of activation energy as a principle of enzymatic catalysis, which state do you think has a lower energy? The substrate-bound occluded state or the empty occluded transporter.
Answer
Most likely the substrate-bound occluded state. The interaction with the substrate facilitates the enclosure. Most likely this reduces the activation barrier for the conformational transition between the alternating states of a transporter.
- Transporters facilitate the movement of hydrophilic molecules through lipid bilayers.
- They are similar to enzymes by forming a complex with the substrate, but use an extreme form of induced fit to enclose the substrate, before releasing it on the other side of the membrane. Transport can be analysed using Michaelis-Menten kinetic algorithms.
- Primary active transporters establish ion gradients across membranes.
- Secondary active transporters use these ion gradients or substrate gradients to move metabolites in and out of cells.
- Secondary active transporters can be uniporters, antiporters, or symporters.
- Uniporters facilitate diffusion along a concentration gradient.
- Symporters mediate accumulation of metabolites against a concentration gradient.
- Antiporters mediate the exchange of metabolites often in the form of a precursor-product exchange.
- Alpha-helices are the dominant secondary structure in transport proteins.
- Flexible parts of a transporter protein move against scaffold domains to provide alternating access.
- LadyofHats Mariana Ruiz [Public domain], via Wikimedia Commons
- By the author using Powerpoint
- By the author using PyMol
- By the author using Powerpoint
- By the author using Powerpoint
- By the author using ChemDraw
- By the author using ChemDraw
- By the author using PyMol